Asia Pac Allergy.
2012 Jan;2(1):49-58. 10.5415/apallergy.2012.2.1.49.
Relationship between sensitivity to dyspnea and fluctuating peak expiratory flow rate in the absence of asthma symptoms
- Affiliations
-
- 1Department of Pulmonary Medicine and Clinical Immunology, Dokkyo Medical University, Mibu, Tochigi 321-0293, Japan. sugiyama@dokkyomed.ac.jp
- KMID: 2397411
- DOI: http://doi.org/10.5415/apallergy.2012.2.1.49
Abstract
- BACKGROUND
Exacerbation of asthma has a negative impact on quality of life and increases the risk of fatal asthma. One of the known risk factors for patients with a history of near-fatal asthma is reduced sensitivity to dyspnea.
OBJECTIVE
We aimed to identify patients with such risk before they experienced severe exacerbation of asthma.
METHODS
We analyzed asthma symptoms and peak expiratory flow rate (PEFR) values of 53 patients recorded daily in a diary over a mean period of 274 days. Patients matched their symptoms to one of eight categories ranging in severity from 'absent' to 'severe attack'. We then analyzed the relationship between PEFR and asthma symptoms by dividing the PEFR value by the values of clinical parameters, including asthma symptom level.
RESULTS
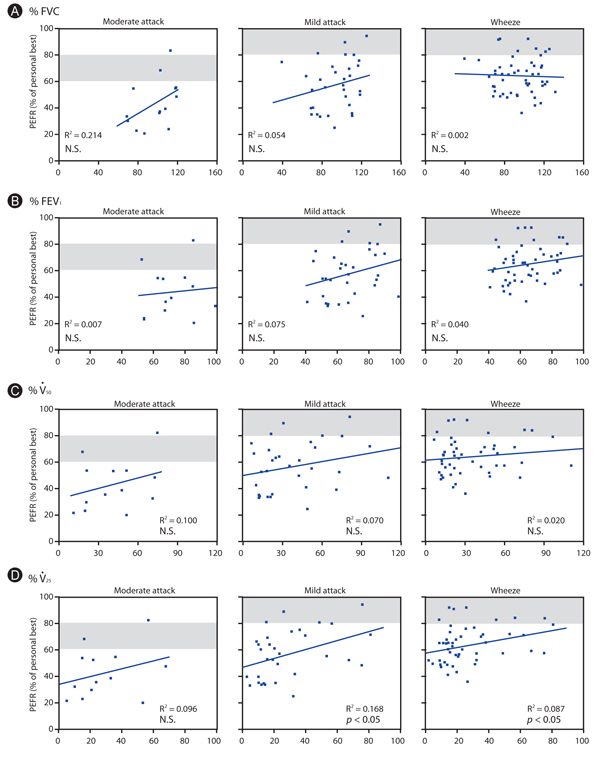
Average PEFR was 75.2% (50.5-100%) in the 'absent' symptom category, 64.5% (36.6-92.6%) in 'wheeze', 57.3% (25.0-94.7%) in 'mild attack' and 43.6% (20.4-83.1%) in 'moderate attack', with the personal best reading taken as 100%. Thus, differences in PEFR in patients in the same symptom category varied widely. PEFR in wheeze, mild attack and moderate attack did not correlate significantly with duration of asthma, forced expiratory volume in one second or proportion of personal best to standard predicted PEFR values. These PEFRs showed no significant difference in groups divided by type of regular treatment, but showed a significant negative correlation with the coefficient of variation (CV) of PEFR when asthma symptoms were absent. CV for absent symptoms should be between +4.0 and -4.0% when using regression analysis to measure PEFR if the decreased PEFR is in agreement with guidelines.
CONCLUSION
To determine which patients have reduced sensitivity to dyspnea, CV of PEFR should be considered when asthma symptoms are reported as absent. When patients present with more than 8% fluctuation in PEFR, we should intervene in their treatment, even when they claim to be stable.
Keyword
MeSH Terms
Figure
Reference
-
1. Gregg I. Can measurement of peak expiratory flow enhance compliance in chronic asthma? Eur Respir J. 1992. 5:136–138.2. Rosenblatt G, Alkalay I, McCann PD, Stein M. The correlation of peak flow rate with maximal expiratory flow rate, one-second forced expiratory volume, and maximal breathing capacity. Am Rev Respir Dis. 1963. 87:589–591.3. Fairbairn AS, Fletcher CM, Tinker CM, Wood CH. A comparison of spirometric and peak expiratory flow measurements in men with and without chronic bronchitis. Thorax. 1962. 17:168–174.
Article4. Troyanov S, Ghezzo H, Cartier A, Malo JL. Comparison of circadian variations using FEV1 and peak expiratory flow rates among normal and asthmatic subjects. Thorax. 1994. 49:775–780.5. Gautrin D, D'Aquino LC, Gagnon G, Malo JL, Cartier A. Comparison between peak expiratory flow rates (PEFR) and FEV1 in the monitoring of asthmatic subjects at an outpatient clinic. Chest. 1994. 106:1419–1426.6. Boutin C, Barré A, Charpin J. Comparison between peak expiratory flow rate and daily report of the symptoms in asthmatic children. Bull Physiopathol Respir (Nancy). 1975. 11:285–294.7. Pauli G, Bigot H, Pelletier A, Kopferschmitt-Kubler MC, Roegel E. A study of the criteria used for clinical evaluation of prophylactic treatment in bronchial asthma. Eur J Respir Dis. 1985. 67:369–377.
Article8. Malo JL, L'Archevêque J, Trudeau C, d'Aquino C, Cartier A. Should we monitor peak expiratory flow rates or record symptoms with a simple diary in the management of asthma? J Allergy Clin Immunol. 1993. 91:702–709.
Article9. Gern JE, Eggleston PA, Schuberth KC, Eney ND, Goldstein EO, Weiss ME, Adkinson NF Jr. Peak flow variation in childhood asthma: a three-year analysis. J Allergy Clin Immunol. 1994. 93:706–716.
Article10. Motojima S, Kurusu H, Fukuda T, Makino S. Management of asthma in use of PEF. Complication with symptom. Ther Res. 1993. 14:3053–3061.11. Expert Panel on the Management of Asthma. Guidelines for the Diagnosis and Management of Asthma: Expert Panel Report. 1991. Bethesda: National Asthma Educational Program, Office of Prevention, Education, and Control, National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services, Public Health Service.12. Asthma Committee of Japanese Society of Allergology. Makino S, editor. Guidelines for diagnosis and management of bronchial asthma. Guidelines for the management of allergic diseases. 1993. Tokyo: Life Science Medica;1–37.13. Japanese Society of Allergology. Committee on the Definition, Treatment, and Management of Bronchial Asthma. Guidelines for the diagnosis and management of bronchial asthma. Allergy. 1995. 50:27 Suppl. 1–42.14. Rubinfeld AR, Pain MC. Perception of asthma. Lancet. 1976. 1:882–884.
Article15. McFadden ER Jr. Fatal and near-fatal asthma. N Engl J Med. 1991. 324:409–411.
Article16. Molfino NA, Nannini LJ, Martelli AN, Slutsky AS. Respiratory arrest in near-fatal asthma. N Engl J Med. 1991. 324:285–288.
Article17. Barnes PJ. Poorly perceived asthma. Thorax. 1992. 47:408–409.
Article18. Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthma. N Engl J Med. 1994. 330:1329–1334.
Article19. Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002. 121:329–333.
Article20. Grampian Asthma Study of Integrated Care (GRASSIC). Effectiveness of routine self monitoring of peak flow in patients with asthma. BMJ. 1994. 308:564–567.21. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2010. Treatment to achieve control. 2010. Geneva: GINA;61–65.22. Yamamura Y, Mitsui S, Sudo M, Takishima T, Kobayashi S, Nemoto T, et al. Classification of severity of asthma according to Japanese Society of Allergology (in Japanese). Jpn J Allergol. 1983. 32:1186–1199.23. Miyamoto T, Shida T, Tomioka H, Makino S, Kabe J, Nakajima S, et al. Classification of severity of asthma according to Japanese Society of Allergology (in Japanese). Jpn J Allergol. 1994. 43:71–80.24. Pagano M, Gauvreau K. Principles of biostatistics. 2000. 2nd ed. Duxbury: Pacific Grove.25. The Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2009. 2009. Bethesda: National Institutes of Health publications.26. Japanese Society of Allergology. Japanese Guidelines for the Diagnosis and Treatment of Allergic Diseases 2010. 2010. Tokyo: Kyowa Kikaku Tsushin.27. Baldwin ED, Cournand A, Richards DW Jr. Pulmonary insufficiency; physiological classification, clinical methods of analysis, standard values in normal subjects. Medicine (Baltimore). 1948. 27:243–278.28. Cherniack RM, Raber MB. Normal standards for ventilatory function using an automated wedge spirometer. Am Rev Respir Dis. 1972. 106:38–46.29. Miyamoto T, Makino S, Toda M, Tsukioka K. Peak expiratory flow in normal, healthy Japanese subjects. 1995. Tokyo: Kyowa Kikaku Tsushin.30. James AL, Paré PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989. 139:242–246.
Article31. Fredberg JJ. Airway smooth muscle in asthma: flirting with disaster. Eur Respir J. 1998. 12:1252–1256.
Article32. Jeffery PK. Pathology of asthma. Br Med Bull. 1992. 48:23–39.
Article33. Dolhnikoff M, da Silva LF, de Araujo BB, Gomes HA, Fernezlian S, Mulder A, Lindeman JH, Mauad T. The outer wall of small airways is a major site of remodeling in fatal asthma. J Allergy Clin Immunol. 2009. 123:1090–1097. 1097.e1
Article34. Brand PL, Postma DS, Kerstjens HA, Koëter GH. The Dutch CNSLD Study Group. Relationship of airway hyperresponsiveness to respiratory symptoms and diurnal peak flow variation in patients with obstructive lung disease. Am Rev Respir Dis. 1991. 143:916–921.
Article35. Hetzel MR, Clark TJ, Branthwaite MA. Asthma: analysis of sudden deaths and ventilatory arrests in hospital. Br Med J. 1977. 1:808–811.
Article36. Sugiyama K, Yamada I, Ohara T, Tatewaki M, Hayashi Y, Arai R, Kamiya K, Fukushima F, Hirata H, Arima M, Fukushima Y, Fukuda T. An analysis of characteristics of patients with exacerbation of asthma in a large university hospital in Japan. Asian Pac J Allergy Immunol. 2010. 28:242–249.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study of predicted values of peak expiratory flow rates in primary school children
- Efficacy and safety of budesonide turbuhaler in Korean asthmatic patients
- Effect of Bedding Control on Amount of House Dust Mite Allergens, Asthma Symptoms, and Peak Expiratory Flow Rate
- "Maximal Expiratory Flow Rates in Pulmonary Diseases" Comparison of the Values at Rest and During and after Exercise
- Comparison of Mini-Wright Peak Flow Meter and Microplus Pocket Spirometer in Measuring Peak Expiratory Flow Rate