Asia Pac Allergy.
2013 Oct;3(4):231-240. 10.5415/apallergy.2013.3.4.231.
CYP1A2 polymorphism and theophylline clearance in Korean non-smoking asthmatics
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul 110-799, Korea. shcho@snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul 110-799, Korea.
- 3Health Insurance Review and Assessment Service, Seoul 137-706, Korea.
- 4Department of Internal Medicine, Dongguk University Medical Center, Goyang 410-773, Korea.
- KMID: 2397300
- DOI: http://doi.org/10.5415/apallergy.2013.3.4.231
Abstract
- BACKGROUND
Theophylline is mainly metabolized by cytochrome P450 (CYP) 1A2 and CYP2E1 which show inter-individual variations. However, the underlying mechanism remains unknown in humans. We investigated the relationship between differences in theophylline clearance and genetic polymorphisms in the CYP1A2 and CYP2E1 gene in 89 Korean asthmatic patients.
METHODS
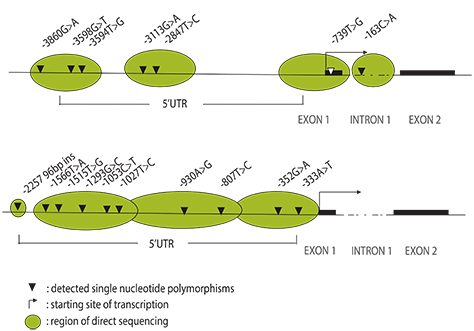
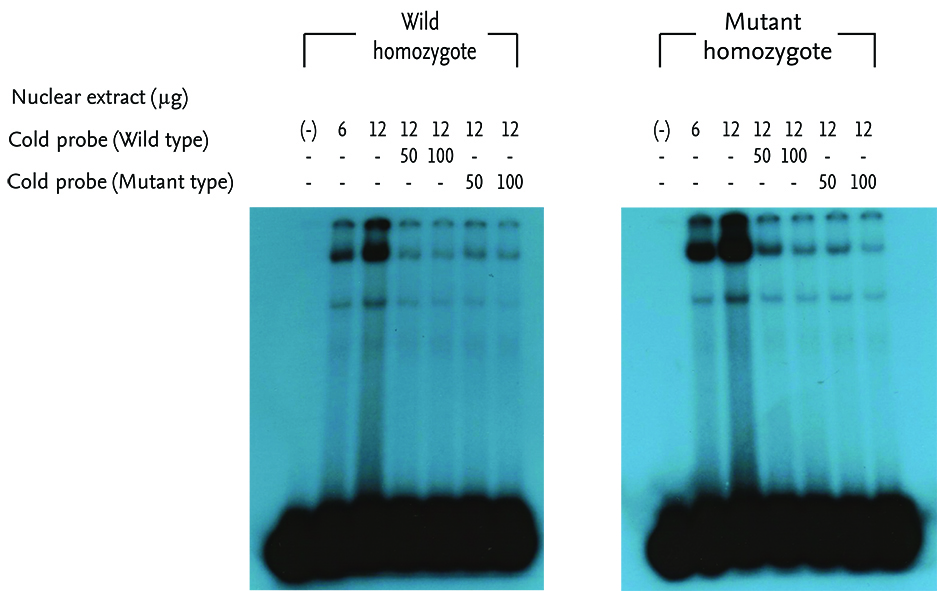
Polymerase chain reaction (PCR) was performed on the 5'-flanking region of those genes. PCR products were directly sequenced and confirmed using the SNaP shot method. We determined whether the detected SNPs affected gene transcription using electrophoretic mobility shift assay (EMSA). Theophylline clearance (mL/kg/h) was assessed by using a Bayesian approach.
RESULTS
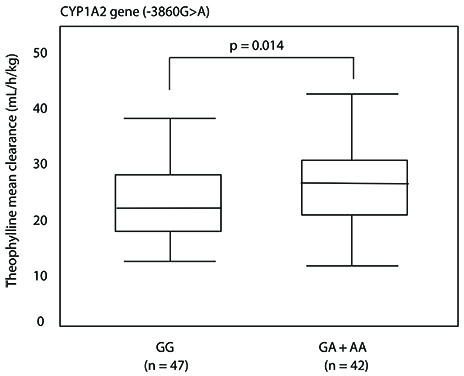
Genetic polymorphisms were identified at 7 sites in the CYP1A2 gene and at 10 sites in the CYP2E1. Among them, subjects with genotypes (GA+AA) of the -3860G>A polymorphism were found to show higher theophylline clearance than those with genotypes GG (29.11 ± 0.91 mL/kg/h vs. 26.12 ± 0.80 mL/kg/h, p = 0.014). This polymorphic site was revealed to be a protein binding site by conducting EMSA on nuclear hepatocyte extracts.
CONCLUSION
In conclusion, increased theophylline clearance was significantly related to the -3860G>A polymorphism, which could be associated with increased CYP1A2 inducibility in Korean non-smoking asthmatics.
Keyword
MeSH Terms
-
Bayes Theorem
Cytochrome P-450 CYP1A2*
Cytochrome P-450 CYP2E1
Cytochrome P-450 Enzyme System
Electrophoretic Mobility Shift Assay
Genotype
Hepatocytes
Humans
Methods
Polymerase Chain Reaction
Polymorphism, Genetic
Polymorphism, Single Nucleotide
Protein Binding
Theophylline*
Cytochrome P-450 CYP1A2
Cytochrome P-450 CYP2E1
Cytochrome P-450 Enzyme System
Theophylline
Figure
Reference
-
1. Mitenko PA, Ogilvie RI. Rational intravenous doses of theophylline. N Engl J Med. 1973; 289:600–603.
Article2. Ellis EF, Koysooko R, Levy G. Pharmacokinetics of theophylline in children with asthma. Pediatrics. 1976; 58:542–547.
Article3. Møller SE, Larsen F, Pitsiu M, Rolan PE. Effect of citalopram on plasma levels of oral theophylline. Clin Ther. 2000; 22:1494–1501.
Article4. Robson RA, Miners JO, Matthews AP, Stupans I, Meller D, McManus ME, Birkett DJ. Characterisation of theophylline metabolism by human liver microsomes. Inhibition and immunochemical studies. Biochem Pharmacol. 1988; 37:1651–1659.5. Grygiel JJ, Wing LM, Farkas J, Birkett DJ. Effects of allopurinol on theophylline metabolism and clearance. Clin Pharmacol Ther. 1979; 26:660–667.
Article6. Rasmussen BB, Maënpää J, Pelkonen O, Loft S, Poulsen HE, Lykkesfeldt J, Brøsen K. Selective serotonin reuptake inhibitors and theophylline metabolism in human liver microsomes: potent inhibition by fluvoxamine. Br J Clin Pharmacol. 1995; 39:151–159.
Article7. Hammons GJ, Guengerich FP, Weis CC, Beland FA, Kadlubar FF. Metabolic oxidation of carcinogenic arylamines by rat, dog, and human hepatic microsomes and by purified flavin-containing and cytochrome P-450 monooxygenases. Cancer Res. 1985; 45:3578–3585.8. Minchin RF, McManus ME, Boobis AR, Davies DS, Thorgeirsson SS. Polymorphic metabolism of the carcinogen 2-acetylaminofluorene in human liver microsomes. Carcinogenesis. 1985; 6:1721–1724.
Article9. Tsutsumi M, Wang JS, Takase S, Takada A. Hepatic messenger RNA contents of cytochrome P4502E1 in patients with different P4502E1 genotypes. Alcohol Alcohol Suppl. 1994; 29:29–32.
Article10. Kalow W, Tang BK. Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase activities. Clin Pharmacol Ther. 1991; 50:508–519.
Article11. Butler MA, Lang NP, Young JF, Caporaso NE, Vineis P, Hayes RB, Teitel CH, Massengill JP, Lawsen MF, Kadlubar FF. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992; 2:116–127.
Article12. Watanabe J, Hayashi S, Kawajiri K. Different regulation and expression of the human CYP2E1 gene due to the RsaI polymorphism in the 5'-flanking region. J Biochem. 1994; 116:321–326.13. Ikeya K, Jaiswal AK, Owens RA, Jones JE, Nebert DW, Kimura S. Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol. 1989; 3:1399–1408.14. Butler MA, Guengerich FP, Kadlubar FF. Metabolic oxidation of the carcinogens 4-aminobiphenyl and 4,4'-methylene-bis(2-chloroaniline) by human hepatic microsomes and by purified rat hepatic cytochrome P-450 monooxygenases. Cancer Res. 1989; 49:25–31.15. Aitchison KJ, Gonzalez FJ, Quattrochi LC, Sapone A, Zhao JH, Zaher H, Elizondo G, Bryant C, Munro J, Collier DA, Makoffa AI, Kerwin RW. Identification of novel polymorphisms in the 5' flanking region of CYP1A2, characterization of interethnic variability, and investigation of their functional significance. Pharmacogenetics. 2000; 10:695–704.
Article16. Hamdy SI, Hiratsuka M, Narahara K, Endo N, El-Enany M, Moursi N, Ahmed MS, Mizugaki M. Genotyping of four genetic polymorphisms in the CYP1A2 gene in the Egyptian population. Br J Clin Pharmacol. 2003; 55:321–324.
Article17. Quattrochi LC, Tukey RH. The human cytochrome Cyp1A2 gene contains regulatory elements responsive to 3-methylcholanthrene. Mol Pharmacol. 1989; 36:66–71.18. Chung I, Bresnick E. Regulation of the constitutive expression of the human CYP1A2 gene: cis elements and their interactions with proteins. Mol Pharmacol. 1995; 47:677–685.19. Obase Y, Shimoda T, Kawano T, Saeki S, Tomari SY, Mitsuta-Izaki K, Matsuse H, Kinoshita M, Kohno S. Polymorphisms in the CYP1A2 gene and theophylline metabolism in patients with asthma. Clin Pharmacol Ther. 2003; 73:468–474.20. Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5'-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem. 1999; 125:803–808.21. Welfare MR, Aitkin M, Bassendine MF, Daly AK. Detailed modelling of caffeine metabolism and examination of the CYP1A2 gene: lack of a polymorphism in CYP1A2 in Caucasians. Pharmacogenetics. 1999; 9:367–375.22. Quattrochi LC, Shih H, Pickwell GV. Induction of the human CYP1A2 enhancer by phorbol ester. Arch Biochem Biophys. 1998; 350:41–48.
Article23. Pfaff DW, Freidin MM, Wu-Peng XS, Yin J, Zhu YS. Competition for DNA steroid response elements as a possible mechanism for neuroendocrine integration. J Steroid Biochem Mol Biol. 1994; 49:373–379.
Article24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.
Article25. Gorski K, Carneiro M, Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986; 47:767–776.
Article26. Tanigawara Y, Komada F, Shimizu T, Iwakawa S, Iwai T, Maekawa H, Hori R, Okumura K. Population pharmacokinetics of theophylline. III. Premarketing study for a once-daily administered preparation. Biol Pharm Bull. 1995; 18:1590–1598.
Article27. Nie H, Zhang G, Liu M, Ding X, Huang Y, Hu S. Efficacy of theophylline plus salmeterol/fluticasone propionate combination therapy in patients with asthma. Respir Med. 2013; 107:347–354.
Article28. Ford PA, Durham AL, Russell RE, Gordon F, Adcock IM, Barnes PJ. Treatment effects of low-dose theophylline combined with an inhaled corticosteroid in COPD. Chest. 2010; 137:1338–1344.
Article29. Ohta K, Fukuchi Y, Grouse L, Mizutani R, Rabe KF, Rennard SI, Zhong NS. A prospective clinical study of theophylline safety in 3810 elderly with asthma or COPD. Respir Med. 2004; 98:1016–1024.
Article30. Yoon Y, Park HD, Park KU, Kim JQ, Chang YS, Song J. Associations between CYP2E1 promoter polymorphisms and plasma 1,3-dimethyluric acid/theophylline ratios. Eur J Clin Pharmacol. 2006; 62:627–631.
Article31. Jennings TS, Nafziger AN, Davidson L, Bertino JS Jr. Gender differences in hepatic induction and inhibition of theophylline pharmacokinetics and metabolism. J Lab Clin Med. 1993; 122:208–216.32. Takata K, Saruwatari J, Nakada N, Nakagawa M, Fukuda K, Tanaka F, Takenaka S, Mihara S, Marubayashi T, Nakagawa K. Phenotypegenotype analysis of CYP1A2 in Japanese patients receiving oral theophylline therapy. Eur J Clin Pharmacol. 2006; 62:23–28.
Article33. Shimoda K, Someya T, Morita S, Hirokane G, Yokono A, Takahashi S, Okawa M. Lack of impact of CYP1A2 genetic polymorphism (C/A polymorphism at position 734 in intron 1 and G/A polymorphism at position -2964 in the 5'-flanking region of CYP1A2) on the plasma concentration of haloperidol in smoking male Japanese with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2002; 26:261–265.
Article34. Mihara K, Suzuki A, Kondo T, Yasui N, Furukori H, Nagashima U, Ono S, Kaneko S, Otani K, Inoue Y. Effect of a genetic polymorphism of CYP1A2 inducibility on the steady state plasma concentrations of haloperidol and reduced haloperidol in Japanese patients with schizophrenia. Ther Drug Monit. 2000; 22:245–249.
Article35. Tantcheva-Poór I, Zaigler M, Rietbrock S, Fuhr U. Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999; 9:131–144.36. Ou-Yang DS, Huang SL, Wang W, Xie HG, Xu ZH, Shu Y, Zhou HH. Phenotypic polymorphism and gender-related differences of CYP1A2 activity in a Chinese population. Br J Clin Pharmacol. 2000; 49:145–151.
Article37. Hayashi S, Watanabe J, Kawajiri K. Genetic polymorphisms in the 5'-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem. 1991; 110:559–565.38. Lee MY, Mukherjee N, Pakstis AJ, Khaliq S, Mohyuddin A, Mehdi SQ, Speed WC, Kidd JR, Kidd KK. Global patterns of variation in allele and haplotype frequencies and linkage disequilibrium across the CYP2E1 gene. Pharmacogenomics J. 2008; 8:349–356.
Article39. Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P, Bouchardy C, Breskvar K, Brockmoller J, Cascorbi I, Clapper ML, Coutelle C, Daly A, Dell'Omo M, Dolzan V, Dresler CM, Fryer A, Haugen A, Hein DW, Hildesheim A, Hirvonen A, Hsieh LL, Ingelman-Sundberg M, Kalina I, Kang D, Kihara M, Kiyohara C, Kremers P, Lazarus P, Le Marchand L, Lechner MC, van Lieshout EM, London S, Manni JJ, Maugard CM, Morita S, Nazar-Stewart V, Noda K, Oda Y, Parl FF, Pastorelli R, Persson I, Peters WH, Rannug A, Rebbeck T, Risch A, Roelandt L, Romkes M, Ryberg D, Salagovic J, Schoket B, Seidegard J, Shields PG, Sim E, Sinnet D, Strange RC, Stücker I, Sugimura H, To-Figueras J, Vineis P, Yu MC, Taioli E. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001; 10:1239–1248.40. Mitchell AA, Zwick ME, Chakravarti A, Cutler DJ. Discrepancies in dbSNP confirmation rates and allele frequency distributions from varying genotyping error rates and patterns. Bioinformatics. 2004; 20:1022–1032.
Article41. Park KS, Mok JW. Polymorphisms of the CYP2E1 in Koreans. Genes Genom. 2002; 24:149–155.42. Lucas D, Ménez C, Girre C, Berthou F, Bodénez P, Joannet I, Hispard E, Bardou LG, Ménez JF. Cytochrome P450 2E1 genotype and chlorzoxazone metabolism in healthy and alcoholic Caucasian subjects. Pharmacogenetics. 1995; 5:298–304.
Article43. Powell H, Kitteringham NR, Pirmohamed M, Smith DA, Park BK. Expression of cytochrome P4502E1 in human liver: assessment by mRNA, genotype and phenotype. Pharmacogenetics. 1998; 8:411–421.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CYP1A2 activity as a risk factor for bladder cancer
- The variation of theophylline clearance with concurrent herb medication
- Pharmacokinetic study of theophylline in Korean adult asthmatics
- Drug Interactions between Theophylline and H2-antagonists; Ranitidine, Famotidine
- Effects of Erythromycin and New Macrolides on the Serum Theophylline Level and Clearance