Asia Pac Allergy.
2015 Apr;5(2):59-67. 10.5415/apallergy.2015.5.2.59.
Recent advances of pharmacogenomics in severe cutaneous adverse reactions: immune and nonimmune mechanisms
- Affiliations
-
- 1Department of Dermatology, Drug Hypersensitivity Clinical and Research Center, Chang Gung Memorial Hospitals, Taipei, Linkou, and Keelung, Taiwan. wenhungchung@yahoo.com
- 2College of Medicine, Chang Gung University, Taoyuan 33302, Taiwan.
- KMID: 2397052
- DOI: http://doi.org/10.5415/apallergy.2015.5.2.59
Abstract
- Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) are severe cutaneous adverse reactions (SCAR) which are majorly caused by drugs. Though the incidence rate is low, SCAR sometimes can be life-threatening and leads to lifelong sequelae. Many pharmacogenomic associations in immune and nonimmune related genes with the development of SCAR have been discovered recently and the pharmacogenetic tests have been applied to prevent specific drug-induced SCAR. In this review, we discuss the recent advances of pharmacogenomics in SCAR.
Keyword
Figure
Cited by 2 articles
-
In this issue of Asia Pacific allergy
Constance H. Katelaris
Asia Pac Allergy. 2015;5(2):57-58. doi: 10.5415/apallergy.2015.5.2.57.Asia Pacific Allergy: it's been five years!
Yoon-Seok Chang
Asia Pac Allergy. 2016;6(1):1-2. doi: 10.5415/apallergy.2016.6.1.1.
Reference
-
1. Bastuji-Garin S, Zahedi M, Guillaume JC, Roujeau JC. Toxic epidermal necrolysis (Lyell syndrome) in 77 elderly patients. Age Ageing. 1993; 22:450–456.
Article2. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994; 331:1272–1285.
Article3. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993; 129:92–96.
Article4. Wong KC, Kennedy PJ, Lee S. Clinical manifestations and outcomes in 17 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Australas J Dermatol. 1999; 40:131–134.
Article5. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994; 102:28S–30S.
Article6. Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schroder W, Roujeau JC. SCAR Study Group. Severe Cutaneous Adverse Reactions. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002; 138:1019–1024.
Article7. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, Roujeau JC. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007; 156:609–611.
Article8. Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996; 15:250–257.
Article9. Chi MH, Hui RC, Yang CH, Lin JY, Lin YT, Ho HC, Chung WH, Kuo TT. Histopathological analysis and clinical correlation of drug reaction with eosinophilia and systemic symptoms (DRESS). Br J Dermatol. 2014; 170:866–873.
Article10. Valeyrie-Allanore L, Sassolas B, Roujeau JC. Drug-induced skin, nail and hair disorders. Drug Saf. 2007; 30:1011–1030.
Article11. Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, Kardaun S, Sidoroff A, Liss Y, Schumacher M, Roujeau JC. RegiSCAR study group. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013; 133:1197–1204.
Article12. Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, Haustein UF, Vieluf D, Roujeau JC, Le Louet H. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010; 88:60–68.
Article13. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, Sidoroff A, Schneck J, Roujeau JC, Flahault A. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008; 128:35–44.
Article14. Halevy S, Ghislain PD, Mockenhaupt M, Fagot JP, Bouwes Bavinck JN, Sidoroff A, Naldi L, Dunant A, Viboud C, Roujeau JC. EuroSCAR Study Group. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008; 58:25–32.
Article15. Levi N, Bastuji-Garin S, Mockenhaupt M, Roujeau JC, Flahault A, Kelly JP, Martin E, Kaufman DW, Maison P. Medications as risk factors of Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009; 123:e297–e304.
Article16. Amante MF, Filippini AV, Cejas N, Lendoire J, Imventarza O, Parisi C. Dress syndrome and fulminant hepatic failure induced by lamotrigine. Ann Hepatol. 2009; 8:75–77.
Article17. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007; 56:181–200.
Article18. Borchers AT, Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Stevens-Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev. 2008; 7:598–605.
Article19. Trent J, Halem M, French LE, Kerdel F. Toxic epidermal necrolysis and intravenous immunoglobulin: a review. Semin Cutan Med Surg. 2006; 25:91–93.
Article20. Revuz J, Penso D, Roujeau JC, Guillaume JC, Payne CR, Wechsler J, Touraine R. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol. 1987; 123:1160–1165.
Article21. Schopf E, Stuhmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol. 1991; 127:839–842.
Article22. Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, Roujeau JC. The DRESS syndrome: a literature review. Am J Med. 2011; 124:588–597.
Article23. Paquet P, Pierard GE. New insights in toxic epidermal necrolysis (Lyell's syndrome): clinical considerations, pathobiology and targeted treatments revisited. Drug Saf. 2010; 33:189–212.24. Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998; 282:490–493.
Article25. Sullivan JR, Watson A. Lamotrigine-induced toxic epidermal necrolysis treated with intravenous cyclosporin: a discussion of pathogenesis and immunosuppressive management. Australas J Dermatol. 1996; 37:208–212.
Article26. Rai R, Srinivas CR. Suprapharmacologic doses of intravenous dexamethasone followed by cyclosporine in the treatment of toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol. 2008; 74:263–265.
Article27. Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol. 2013; 79:686–692.
Article28. Lissia M, Mulas P, Bulla A, Rubino C. Toxic epidermal necrolysis (Lyell's disease). Burns. 2010; 36:152–163.
Article29. Fromowitz JS, Ramos-Caro FA, Flowers FP. University of Florida. Practical guidelines for the management of toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 2007; 46:1092–1094.
Article30. Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008; 58:33–40.
Article31. Mittmann N, Chan B, Knowles S, Cosentino L, Shear N. Intravenous immunoglobulin use in patients with toxic epidermal necrolysis and Stevens-Johnson syndrome. Am J Clin Dermatol. 2006; 7:359–368.
Article32. Murata J, Abe R, Shimizu H. Increased soluble Fas ligand levels in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis preceding skin detachment. J Allergy Clin Immunol. 2008; 122:992–1000.
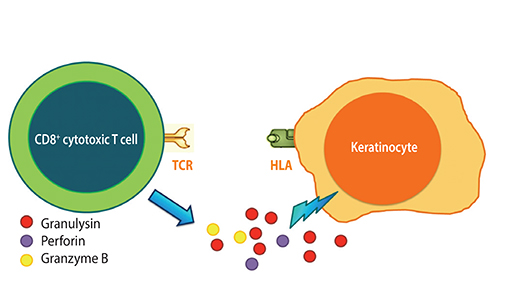
Article33. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, Yang CH, Lu CF, Wu JY, Liao YD, Chen YT. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008; 14:1343–1350.
Article34. Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol. 1998; 161:1758–1764.35. Deng A, Chen S, Li Q, Lyu SC, Clayberger C, Krensky AM. Granulysin, a cytolytic molecule, is also a chemoattractant and proinflammatory activator. J Immunol. 2005; 174:5243–5248.
Article36. Pichler WJ. Pharmacological interaction of drugs with antigenspecific immune receptors: the p-i concept. Curr Opin Allergy Clin Immunol. 2002; 2:301–305.
Article37. Naisbitt DJ, Britschgi M, Wong G, Farrell J, Depta JP, Chadwick DW, Pichler WJ, Pirmohamed M, Park BK. Hypersensitivity reactions to carbamazepine: characterization of the specificity, phenotype, and cytokine profile of drug-specific T cell clones. Mol Pharmacol. 2003; 63:732–741.
Article38. David V, Bourge JF, Guglielmi P, Mathieu-Mahul D, Degos L, Bensussan A. Human T cell clones use a CD3-associated surface antigen recognition structure to exhibit both NK-like and allogeneic cytotoxic reactivity. J Immunol. 1987; 138:2831–2836.39. Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, Chen YT, Hung SI. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011; 128:1266–1276.e11.
Article40. Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012; 129:1562–1569.e5.
Article41. Roujeau JC, Huynh TN, Bracq C, Guillaume JC, Revuz J, Touraine R. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 1987; 123:1171–1173.
Article42. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jagel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A. PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008; 358:568–579.43. Puthanakit T, Bunupuradah T, Kosalaraksa P, Vibol U, Hansudewechakul R, Ubolyam S, Suwanlerk T, Kanjanavanit S, Ngampiyaskul C, Wongsawat J, Luesomboon W, Vonthanak S, Ananworanich J, Ruxrungtham K. PREDICT Study Group. Prevalence of human leukocyte antigen-B*5701 among HIV-infected children in Thailand and Cambodia: implications for abacavir use. Pediatr Infect Dis J. 2013; 32:252–253.44. Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, Lan JL, Yang LC, Hong HS, Chen MJ, Lai PC, Wu MS, Chu CY, Wang KH, Chen CH, Fann CS, Wu JY, Chen YT. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005; 102:4134–4139.45. Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, Chucherd P, Konyoung P, Vannaprasaht S, Choonhakarn C, Pisuttimarn P, Sangviroon A, Tassaneeyakul W. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009; 19:704–709.46. Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, Sawada J, Furuya H, Takahashi Y, Muramatsu M, Kinoshita S, Abe M, Ikeda H, Kashiwagi M, Song Y, Ueta M, Sotozono C, Ikezawa Z, Hasegawa R. JSAR research group. HLA-B locus in Japanese patients with antiepileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008; 9:1617–1622.
Article47. Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, Park HW, Chang YS, Jang IJ, Cho SH, Min KU, Kim SH, Lee KW. Adverse Drug Reaction Research Group in Korea. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011; 21:303–307.
Article48. Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, Naldi L, Bouwes-Bavinck JN, Sidoroff A, de Toma C, Schumacher M, Roujeau JC, Hovnanian A, Mockenhaupt M. RegiSCAR study group. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008; 18:99–107.
Article49. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004; 428:486.50. Man CB, Kwan P, Baum L, Yu E, Lau KM, Cheng AS, Ng MH. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007; 48:1015–1018.51. Locharernkul C, Loplumlert J, Limotai C, Korkij W, Desudchit T, Tongkobpetch S, Kangwanshiratada O, Hirankarn N, Suphapeetiporn K, Shotelersuk V. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008; 49:2087–2091.52. Mehta TY, Prajapati LM, Mittal B, Joshi CG, Sheth JJ, Patel DB, Dave DM, Goyal RK. Association of HLA-B*1502 allele and carbamazepineinduced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol. 2009; 75:579–582.53. Tangamornsuksan W, Chaiyakunapruk N, Somkrua R, Lohitnavy M, Tassaneeyakul W. Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2013; 149:1025–1032.54. Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, Furuya H, Takahashi Y, Muramatsu M, Kinoshita S, Abe M, Ikeda H, Kashiwagi M, Song Y, Ueta M, Sotozono C, Ikezawa Z, Hasegawa R. JSAR research group. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010; 51:2461–2465.55. Kim SH, Lee KW, Song WJ, Kim SH, Jee YK, Lee SM, Kang HR, Park HW, Cho SH, Park SH, Min KU, Chang YS. Adverse Drug Reaction Research Group in Korea. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011; 97:190–197.
Article56. Shi YW, Min FL, Qin B, Zou X, Liu XR, Gao MM, Wang Q, Zhou JQ, Liao WP. Association between HLA and Stevens-Johnson syndrome induced by carbamazepine in Southern Han Chinese: genetic markers besides B*1502? Basic Clin Pharmacol Toxicol. 2012; 111:58–64.57. Hsiao YH, Hui RC, Wu T, Chang WC, Hsih MS, Yang CH, Ho HC, Chang YG, Chen MJ, Lin JY, Chen DP, Chang PY, Wu TL, Hung SI, Chung WH. Genotype-phenotype association between HLA and carbamazepineinduced hypersensitivity reactions: strength and clinical correlations. J Dermatol Sci. 2014; 73:101–109.
Article58. Ikeda H, Takahashi Y, Yamazaki E, Fujiwara T, Kaniwa N, Saito Y, Aihara M, Kashiwagi M, Muramatsu M. HLA class I markers in Japanese patients with carbamazepine-induced cutaneous adverse reactions. Epilepsia. 2010; 51:297–300.
Article59. Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, Ikezawa Z, Iijima M, Shiohara T, Hashimoto K, Kamatani N, Nakamura Y. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011; 20:1034–1041.60. Niihara H, Kakamu T, Fujita Y, Kaneko S, Morita E. HLA-A31 strongly associates with carbamazepine-induced adverse drug reactions but not with carbamazepine-induced lymphocyte proliferation in a Japanese population. J Dermatol. 2012; 39:594–601.
Article61. Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, Hu SL, Wu MT, Chen GS, Wong TW, Hsiao PF, Chen WH, Shih HY, Fang WH, Wei CY, Lou YH, Huang YL, Lin JJ, Chen YT. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006; 16:297–306.
Article62. Genin E, Chen DP, Hung SI, Sekula P, Schumacher M, Chang PY, Tsai SH, Wu TL, Bellon T, Tamouza R, Fortier C, Toubert A, Charron D, Hovnanian A, Wolkenstein P, Chung WH, Mockenhaupt M, Roujeau JC. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: an international study and metaanalysis. Pharmacogenomics J. 2014; 14:281–288.63. McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciutė D, Carrington M, Sills GJ, Marson T, Jia X, de Bakker PI, Chinthapalli K, Molokhia M, Johnson MR, O'Connor GD, Chaila E, Alhusaini S, Shianna KV, Radtke RA, Heinzen EL, Walley N, Pandolfo M, Pichler W, Park BK, Depondt C, Sisodiya SM, Goldstein DB, Deloukas P, Delanty N, Cavalleri GL, Pirmohamed M. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011; 364:1134–1143.64. Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, Yu YX, Chen MF, Low HQ, Li JH, Bao FF, Foo JN, Bei JX, Jia XM, Liu J, Liany H, Wang N, Niu GY, Wang ZZ, Shi BQ, Tian HQ, Liu HX, Ma SS, Zhou Y, You JB, Yang Q, Wang C, Chu TS, Liu DC, Yu XL, Sun YH, Ning Y, Wei ZH, Chen SL, Chen XC, Zhang ZX, Liu YX, Pulit SL, Wu WB, Zheng ZY, Yang RD, Long H, Liu ZS, Wang JQ, Li M, Zhang LH, Wang H, Wang LM, Xiao P, Li JL, Huang ZM, Huang JX, Li Z, Liu J, Xiong L, Yang J, Wang XD, Yu DB, Lu XM, Zhou GZ, Yan LB, Shen JP, Zhang GC, Zeng YX, de Bakker PI, Chen SM, Liu JJ. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med. 2013; 369:1620–1628.65. Cheung YK, Cheng SH, Chan EJ, Lo SV, Ng MH, Kwan P. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia. 2013; 54:1307–1314.
Article66. Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, Chu CY, Chen YT. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010; 11:349–356.
Article67. Kim SH, Kim M, Lee KW, Kim SH, Kang HR, Park HW, Jee YK. HLAB* 5901 is strongly associated with methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics. 2010; 11:879–884.68. Martin AM, Nolan D, James I, Cameron P, Keller J, Moore C, Phillips E, Christiansen FT, Mallal S. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005; 19:97–99.69. Vitezica ZG, Milpied B, Lonjou C, Borot N, Ledger TN, Lefebvre A, Hovnanian A. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008; 22:540–541.70. Littera R, Carcassi C, Masala A, Piano P, Serra P, Ortu F, Corso N, Casula B, La Nasa G, Contu L, Manconi PE. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006; 20:1621–1626.
Article71. Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, Tantisiriwat W, Charoenyingwattana A, Sura T, Chantratita W, Nakamura Y. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIVinfected Thai patients. Pharmacogenet Genomics. 2009; 19:139–146.72. Gatanaga H, Yazaki H, Tanuma J, Honda M, Genka I, Teruya K, Tachikawa N, Kikuchi Y, Oka S. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS. 2007; 21:264–265.
Article73. Song JS, Kang ES, Joo EY, Hong SB, Seo DW, Lee SY. Absence of HLAB* 1502 and HLA-A*3101 alleles in 9 Korean patients with antiepileptic drug-induced skin rash: a preliminary study. Ann Lab Med. 2014; 34:372–375.74. Ferrell PB Jr, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008; 9:1543–1546.75. Chung WH, Hung SI, Chen YT. Genetic predisposition of life-threatening antiepileptic-induced skin reactions. Expert Opin Drug Saf. 2010; 9:15–21.
Article76. Saito N, Qiao H, Yanagi T, Shinkuma S, Nishimura K, Suto A, Fujita Y, Suzuki S, Nomura T, Nakamura H, Nagao K, Obuse C, Shimizu H, Abe R. An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med. 2014; 6:245ra95.
Article77. Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, Ho HC, Chen MJ, Lin JY, Hui RC, Ho JC, Wu WM, Chen TJ, Wu T, Wu YR, Hsih MS, Tu PH, Chang CN, Hsu CN, Wu TL, Choon SE, Hsu CK, Chen DY, Liu CS, Lin CY, Kaniwa N, Saito Y, Takahashi Y, Nakamura R, Azukizawa H, Shi Y, Wang TH, Chuang SS, Tsai SF, Chang CJ, Chang YS, Hung SI. Taiwan Severe Cutaneous Adverse Reaction Consortium. Japan Pharmacogenomics Data Science Consortium. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014; 312:525–534.
Article78. Kesavan R, Narayan SK, Adithan C. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on phenytoin-induced neurological toxicity in Indian epileptic patients. Eur J Clin Pharmacol. 2010; 66:689–696.
Article79. Depondt C, Godard P, Espel RS, Da Cruz AL, Lienard P, Pandolfo M. A candidate gene study of antiepileptic drug tolerability and efficacy identifies an association of CYP2C9 variants with phenytoin toxicity. Eur J Neurol. 2011; 18:1159–1164.80. Lee AY, Kim MJ, Chey WY, Choi J, Kim BG. Genetic polymorphism of cytochrome P450 2C9 in diphenylhydantoin-induced cutaneous adverse drug reactions. Eur J Clin Pharmacol. 2004; 60:155–159.81. Ciccacci C, Di Fusco D, Marazzi MC, Zimba I, Erba F, Novelli G, Palombi L, Borgiani P, Liotta G. Association between CYP2B6 polymorphisms and Nevirapine-induced SJS/TEN: a pharmacogenetics study. Eur J Clin Pharmacol. 2013; 69:1909–1916.
Article82. Emmerson BT. The management of gout. N Engl J Med. 1996; 334:445–451.
Article83. Nuki G. An appraisal of the 2012 American College of Rheumatology guidelines for the management of gout. Curr Opin Rheumatol. 2014; 26:152–161.
Article84. Chung WH, Chang WC, Stocker SL, Juo CG, Graham GG, Lee MH, Williams KM, Tian YC, Juan KC, Jan Wu YJ, Yang CH, Chang CJ, Lin YJ, Day RO, Hung SI. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis. 2014; 08. 12. [Epub] http://dx.doi.org/10.1136/annrheumdis-2014-205577.
Article