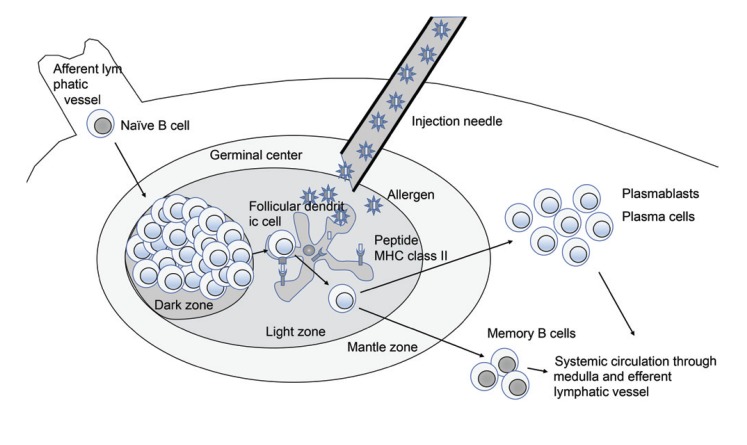
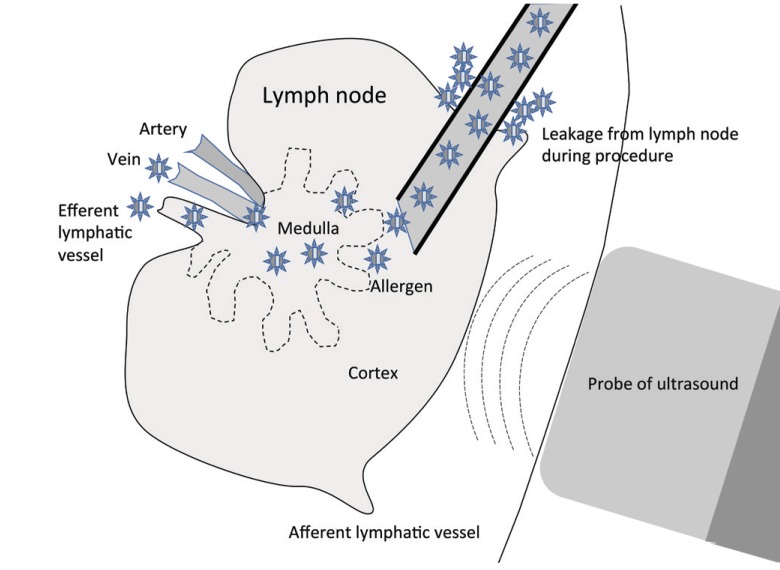
Asia Pac Allergy.
2017 Jul;7(3):131-137. 10.5415/apallergy.2017.7.3.131.
Allergen-specific intralymphatic immunotherapy in human and animal studies
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Gachon University Gil Medical Center, Incheon 21565, Korea.
- 2Department of Radiology, Gachon University Gil Medical Center, Incheon 21565, Korea.
- 3Division of Pulmonology and Allergy, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon 21565, Korea. sangminlee77@naver.com
- KMID: 2396936
- DOI: http://doi.org/10.5415/apallergy.2017.7.3.131
Abstract
- Clinical trials of intralymphatic immunotherapy (ILIT) have been performed to overcome the limitations of long-term therapy and the local or systemic hypersensitivity reactions in conventional allergen-specific immunotherapy, including subcutaneous or sublingual immunotherapy. Additionally, several animal studies of ILIT have been conducted in the form of translational or veterinary research. We conducted a literature review to examine the treatment efficacy and adverse effects of ILIT.
MeSH Terms
Figure
Cited by 3 articles
-
Dog and Cat Allergies and Allergen Avoidance Measures in Korean Adult Pet Owners Who Participated in a Pet Exhibition
Min Suk Yang, Sang Pyo Lee, Young Jae Kwon, Sang Min Lee
Allergy Asthma Immunol Res. 2018;10(2):155-164. doi: 10.4168/aair.2018.10.2.155.Novel strategies in immunotherapy for allergic diseases
Mohana Rajakulendran, Elizabeth Huiwen Tham, Jian Yi Soh, HP Van Bever
Asia Pac Allergy. 2018;8(2):e14. doi: 10.5415/apallergy.2018.8.e14.Novel strategies in immunotherapy for allergic diseases
Mohana Rajakulendran, Elizabeth Huiwen Tham, Jian Yi Soh, HP Van Bever
Asia Pac Allergy. 2018;8(2):. doi: 10.5415/apallergy.2018.8.e14.
Reference
-
1. Kündig TM, Bachmann MF, DiPaolo C, Simard JJ, Battegay M, Lother H, Gessner A, Kühlcke K, Ohashi PS, Hengartner H, et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 1995; 268:1343–1347. PMID: 7761853.
Article2. Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kündig T, Hengartner H. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997; 156:199–209. PMID: 9176709.
Article3. Zinkernagel RM. Localization dose and time of antigens determine immune reactivity. Semin Immunol. 2000; 12:163–171. PMID: 10910735.
Article4. Senti G, Johansen P, Kündig TM. Intralymphatic immunotherapy. Curr Opin Allergy Clin Immunol. 2009; 9:537–543. PMID: 19680119.
Article5. Senti G, Johansen P, Kündig TM. Intralymphatic immunotherapy: from the rationale to human applications. Curr Top Microbiol Immunol. 2011; 352:71–84. PMID: 21725898.
Article6. Senti G, Kündig TM. Intralymphatic immunotherapy. World Allergy Organ J. 2015; 8:9. PMID: 25780493.
Article7. Senti G, Kündig TM. Novel delivery routes for allergy immunotherapy: intralymphatic, epicutaneous, and intradermal. Immunol Allergy Clin North Am. 2016; 36:25–37. PMID: 26617225.8. von Moos S, Kündig TM, Senti G. Novel administration routes for allergen-specific immunotherapy: a review of intralymphatic and epicutaneous allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2011; 31:391–406. xiPMID: 21530827.
Article9. Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, McCormack SJ, Simard JJ, Wüthrich B, Crameri R, Graf N, Johansen P, Kündig TM. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008; 105:17908–17912. PMID: 19001265.
Article10. Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, Steiner M, Hothorn LA, Grönlund H, Tivig C, Zaleska A, Soyer O, van Hage M, Akdis CA, Akdis M, Rose H, Kündig TM. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012; 129:1290–1296. PMID: 22464647.
Article11. Hylander T, Latif L, Petersson-Westin U, Cardell LO. Intralymphatic allergen-specific immunotherapy: an effective and safe alternative treatment route for pollen-induced allergic rhinitis. J Allergy Clin Immunol. 2013; 131:412–420. PMID: 23374268.
Article12. Hylander T, Larsson O, Petersson-Westin U, Eriksson M, Kumlien Georén S, Winqvist O, Cardell LO. Intralymphatic immunotherapy of pollen-induced rhinoconjunctivitis: a double-blind placebo-controlled trial. Respir Res. 2016; 17:10. PMID: 26817454.
Article13. Witten M, Malling HJ, Blom L, Poulsen BC, Poulsen LK. Is intralymphatic immunotherapy ready for clinical use in patients with grass pollen allergy? J Allergy Clin Immunol. 2013; 132:1248–1252.e5. PMID: 24035151.
Article14. Patterson AM, Bonny AE, Shiels WE 2nd, Erwin EA. Three-injection intralymphatic immunotherapy in adolescents and young adults with grass pollen rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2016; 116:168–170. PMID: 26706294.
Article15. Schmid JM, Nezam H, Madsen HH, Schmitz A, Hoffmann HJ. Intralymphatic immunotherapy induces allergen specific plasmablasts and increases tolerance to skin prick testing in a pilot study. Clin Transl Allergy. 2016; 6:19. PMID: 27231527.
Article16. Lee SP, Choi SJ, Joe E, Lee SM, Lee MW, Shim JW, Kim YJ, Kyung SY, Park JW, Jeong SH, Jung JH. A pilot study of intralymphatic immunotherapy for house dust mite, cat, and dog allergies. Allergy Asthma Immunol Res. 2017; 9:272–277. PMID: 28293934.
Article17. Martínez-Gómez JM, Johansen P, Erdmann I, Senti G, Crameri R, Kündig TM. Intralymphatic injections as a new administration route for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2009; 150:59–65. PMID: 19339803.
Article18. Jonsdottir S, Hamza E, Janda J, Rhyner C, Meinke A, Marti E, Svansson V, Torsteinsdottir S. Developing a preventive immunization approach against insect bite hypersensitivity using recombinant allergens: a pilot study. Vet Immunol Immunopathol. 2015; 166:8–21. PMID: 26004943.
Article19. Jonsdottir S, Svansson V, Stefansdottir SB, Schüpbach G, Rhyner C, Marti E, Torsteinsdottir S. A preventive immunization approach against insect bite hypersensitivity: Intralymphatic injection with recombinant allergens in Alum or Alum and monophosphoryl lipid A. Vet Immunol Immunopathol. 2016; 172:14–20. PMID: 27032498.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intralymphatic allergen-specific immunotherapy
- Recent Advances in Allergen-Specific Immunotherapy in Humans: A Systematic Review
- Allergen Specific Immunotherapy in Allergic Rhinitis
- Allergen specific IgG antibodies in nasal secretion during allergen specific immunotherapy
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex