Asia Pac Allergy.
2017 Apr;7(2):65-73. 10.5415/apallergy.2017.7.2.65.
The role of vitamin D in allergic rhinitis
- Affiliations
-
- 1Department of Otorhinolaryngology, The First Affiliated Hospital, Nanjing Medical University, Nanjing, China. chenglei@jsph.org.cn
- 2International Centre for Allergy Research, Nanjing Medical University, Nanjing, China.
- KMID: 2396927
- DOI: http://doi.org/10.5415/apallergy.2017.7.2.65
Abstract
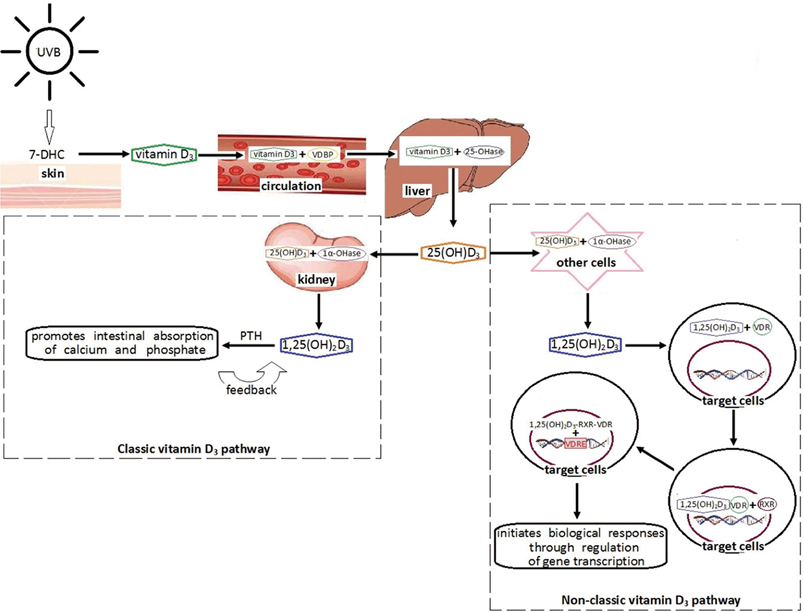
- Recent studies suggest that vitamin D is related to allergic rhinitis (AR). In this review, we first discuss the physiology and metabolism of vitamin D, then we review the function of vitamin D in the immune system, and above all, we highlight the current research regarding the role of vitamin D in AR. Finally, we find that there are both experimental and clinical studies showing that vitamin D is associated with AR, although the results are not consistent and even conflicting. Evidences from those clinical studies show a slightly tendency that serum vitamin D level might be inversely associated with the risk of AR. Meanwhile, it seems that gender and age may influence the relationship between vitamin D and AR. However, because of the heterogeneity in defining AR, differences in study design and so on, all these findings need to be confirmed by further studies. Additional clinical studies as well as experimental research are needed to better understand how vitamin D influences AR.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Intranasal Treatment With 1, 25-Dihydroxyvitamin D3 Alleviates Allergic Rhinitis Symptoms in a Mouse Model
Sung-Woo Cho, Yu-Lian Zhang, Young Kyung Ko, Jae Min Shin, Jun Ho Lee, Chae-Seo Rhee, Dong-Young Kim
Allergy Asthma Immunol Res. 2019;11(2):267-279. doi: 10.4168/aair.2019.11.2.267.Serum 25-hydroxyvitamin D inversely associated with blood eosinophils in patients with persistent allergic rhinitis
Hai-Yan Wu, Jin-Xiang Chen, Hui-Qin Tian, Xiu-Ling Zhang, Hai-Yan Bian, Lei Cheng
Asia Pac Allergy. 2017;7(4):213-220. doi: 10.5415/apallergy.2017.7.4.213.
Reference
-
1. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004; 24:758–764.
Article2. Zhang L, Han D, Huang D, Wu Y, Dong Z, Xu G, Kong W, Bachert C. Prevalence of self-reported allergic rhinitis in eleven major cities in china. Int Arch Allergy Immunol. 2009; 149:47–57.
Article3. Cheng L, Chen YZ. ARIA expert panel. Allergic Rhinitis and its Impact on Asthma (ARIA) achievements in 10 years and future needs. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012; 47:619–622.4. Bousquet J, Schünemann HJ, Samolinski B, Demoly P, Baena-Cagnani CE, Bachert C, Bonini S, Boulet LP, Bousquet PJ, Brozek JL, Canonica GW, Casale TB, Cruz AA, Fokkens WJ, Fonseca JA, van Wijk RG, Grouse L, Haahtela T, Khaltaev N, Kuna P, Lockey RF, Lodrup Carlsen KC, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Palkonen S, Papadopoulos NG, Passalacqua G, Pawankar R, Price D, Ryan D, Simons FE, Togias A, Williams D, Yorgancioglu A, Yusuf OM, Aberer W, Adachi M, Agache I, Aït-Khaled N, Akdis CA, Andrianarisoa A, Annesi-Maesano I, Ansotegui IJ, Baiardini I, Bateman ED, Bedbrook A, Beghé B, Beji M, Bel EH, Ben Kheder A, Bennoor KS, Bergmann KC, Berrissoul F, Bieber T, Bindslev Jensen C, Blaiss MS, Boner AL, Bouchard J, Braido F, Brightling CE, Bush A, Caballero F, Calderon MA, Calvo MA, Camargos PA, Caraballo LR, Carlsen KH, Carr W, Cepeda AM, Cesario A, Chavannes NH, Chen YZ, Chiriac AM, Chivato Pérez T, Chkhartishvili E, Ciprandi G, Costa DJ, Cox L, Custovic A, Dahl R, Darsow U, De Blay F, Deleanu D, Denburg JA, Devillier P, Didi T, Dokic D, Dolen WK, Douagui H, Dubakiene R, Durham SR, Dykewicz MS, El-Gamal Y, El-Meziane A, Emuzyte R, Fiocchi A, Fletcher M, Fukuda T, Gamkrelidze A, Gereda JE, González Diaz S, Gotua M, Guzmán MA, Hellings PW, Hellquist-Dahl B, Horak F, Hourihane JO, Howarth P, Humbert M, Ivancevich JC, Jackson C, Just J, Kalayci O, Kaliner MA, Kalyoncu AF, Keil T, Keith PK, Khayat G, Kim YY, Koffi N'goran B, Koppelman GH, Kowalski ML, Kull I, Kvedariene V, Larenas-Linnemann D, Le LT, Lemière C, Li J, Lieberman P, Lipworth B, Mahboub B, Makela MJ, Martin F, Marshall GD, Martinez FD, Masjedi MR, Maurer M, Mavale-Manuel S, Mazon A, Melen E, Meltzer EO, Mendez NH, Merk H, Mihaltan F, Mohammad Y, Morais-Almeida M, Muraro A, Nafti S, Namazova-Baranova L, Nekam K, Neou A, Niggemann B, Nizankowska-Mogilnicka E, Nyembue TD, Okamoto Y, Okubo K, Orru MP, Ouedraogo S, Ozdemir C, Panzner P, Pali-Schöll I, Park HS, Pigearias B, Pohl W, Popov TA, Postma DS, Potter P, Rabe KF, Ratomaharo J, Reitamo S, Ring J, Roberts R, Rogala B, Romano A, Roman Rodriguez M, Rosado-Pinto J, Rosenwasser L, Rottem M, Sanchez-Borges M, Scadding GK, Schmid-Grendelmeier P, Sheikh A, Sisul JC, Solé D, Sooronbaev T, Spicak V, Spranger O, Stein RT, Stoloff SW, Sunyer J, Szczeklik A, Todo-Bom A, Toskala E, Tremblay Y, Valenta R, Valero AL, Valeyre D, Valiulis A, Valovirta E, Van Cauwenberge P, Vandenplas O, van Weel C, Vichyanond P, Viegi G, Wang DY, Wickman M, Wöhrl S, Wright J, Yawn BP, Yiallouros PK, Zar HJ, Zernotti ME, Zhong N, Zidarn M, Zuberbier T, Burney PG, Johnston SL, Warner JO. World Health Organization Collaborating Center for Asthma and Rhinitis. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012; 130:1049–1062.
Article5. Akbar NA, Zacharek MA. Vitamin D: immunomodulation of asthma, allergic rhinitis, and chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2011; 19:224–228.
Article6. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008; 88:491S–499S.
Article7. Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012; 523:123–133.
Article8. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl). 2010; 88:441–450.
Article9. Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003; 102:3314–3316.10. Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984; 224:1438–1440.11. Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest. 1984; 74:1451–1455.
Article12. Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci. 2014; 1317:70–75.13. Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabryšová L, Christensen J, Gupta A, Saglani S, Bush A, O'Garra A, Brown Z, Hawrylowicz CM. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur J Immunol. 2012; 42:2697–2708.14. Zhang H, Shih DQ, Zhang X. Mechanisms underlying effects of 1,25-Dihydroxyvitamin D3 on the Th17 cells. Eur J Microbiol Immunol (Bp). 2013; 3:237–240.15. Hamzaoui A, Berraïes A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014; 219:873–879.
Article16. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001; 167:4974–4980.17. Vasiliou JE, Lui S, Walker SA, Chohan V, Xystrakis E, Bush A, Hawrylowicz CM, Saglani S, Lloyd CM. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014; 69:1380–1389.18. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007; 179:1634–1647.19. Heine G, Anton K, Henz BM, Worm M. 1alpha,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002; 32:3395–3404.20. Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000; 164:4443–4451.21. Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012; 280:36–43.
Article22. Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012; 217:1292–1300.
Article23. Morán-Auth Y, Penna-Martinez M, Shoghi F, Ramos-Lopez E, Badenhoop K. Vitamin D status and gene transcription in immune cells. J Steroid Biochem Mol Biol. 2013; 136:83–85.
Article24. Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004; 89-90:437–441.
Article25. D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998; 101:252–262.26. Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998; 28:3017–3030.27. Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009; 45:190–197.28. Uysalol M, Mutlu LC, Saracoglu GV, Karasu E, Guzel S, Kayaoglu S, Uzel N. Childhood asthma and vitamin D deficiency in Turkey: is there cause and effect relationship between them? Ital J Pediatr. 2013; 39:78.
Article29. Aldubi HM, Alissa EM, Kamfar HZ, Gaber O, Marzouki ZM. Bronchial asthma and hypovitaminosis D in Saudi children. Asia Pac Allergy. 2015; 5:103–113.
Article30. Confino-Cohen R, Brufman I, Goldberg A, Feldman BS. Vitamin D, asthma prevalence and asthma exacerbations: a large adult population-based study. Allergy. 2014; 69:1673–1680.
Article31. Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016; 71:1001–1009.
Article32. Kerley CP, Hutchinson K, Cormican L, Faul J, Greally P, Coghlan D, Elnazir B. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016; 27:404–412.33. Cassim R, Russell MA, Lodge CJ, Lowe AJ, Koplin JJ, Dharmage SC. The role of circulating 25 hydroxyvitamin D in asthma: a systematic review. Allergy. 2015; 70:339–354.
Article34. Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, Knight J, Gilchrist C, Camargo CA Jr. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy. 2016; 71:1325–1334.
Article35. Heimbeck I, Wjst M, Apfelbacher CJ. Low vitamin D serum level is inversely associated with eczema in children and adolescents in Germany. Allergy. 2013; 68:906–910.
Article36. Wegienka G, Havstad S, Zoratti EM, Kim H, Ownby DR, Johnson CC. Association between vitamin D levels and allergy-related outcomes vary by race and other factors. J Allergy Clin Immunol. 2015; 136:1309–1314.
Article37. Abd-Allah SH, Pasha HF, Hagrass HA, Alghobashy AA. Vitamin D status and vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Egyptian children. Gene. 2014; 536:430–434.
Article38. Mandal M, Tripathy R, Panda AK, Pattanaik SS, Dakua S, Pradhan AK, Chakraborty S, Ravindran B, Das BK. Vitamin D levels in Indian systemic lupus erythematosus patients: association with disease activity index and interferon alpha. Arthritis Res Ther. 2014; 16:R49.
Article39. Zakeri Z, Sandoughi M, Mashhadi MA, Raeesi V, Shahbakhsh S. Serum vitamin D level and disease activity in patients with recent onset rheumatoid arthritis. Int J Rheum Dis. 2016; 19:343–347.
Article40. Osguthorpe JD. Pathophysiology of and potential new therapies for allergic rhinitis. Int Forum Allergy Rhinol. 2013; 3:384–392.
Article41. Hyppönen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Järvelinb MR. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004; 1037:84–95.
Article42. Wjst M, Hyppönen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007; 62:1085–1086.
Article43. Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Camargo CA Jr, Gillman MW, Gold DR, Litonjua AA. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J Allergy Clin Immunol. 2016; 137:1063–1070.e1-2.
Article44. Dogru M, Suleyman A. Serum 25-hydroxyvitamin D3 levels in children with allergic or nonallergic rhinitis. Int J Pediatr Otorhinolaryngol. 2016; 80:39–42.
Article45. Bener A, Ehlayel MS, Bener HZ, Hamid Q. The impact of Vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: An emerging public health problem. J Family Community Med. 2014; 21:154–161.
Article46. Jung JW, Kim JY, Cho SH, Choi BW, Min KU, Kang HR. Allergic rhinitis and serum 25-hydroxyvitamin D level in Korean adults. Ann Allergy Asthma Immunol. 2013; 111:352–357.
Article47. Arshi S, Ghalehbaghi B, Kamrava SK, Aminlou M. Vitamin D serum levels in allergic rhinitis: any difference from normal population? Asia Pac Allergy. 2012; 2:45–48.
Article48. Mai XM, Chen Y, Camargo CA Jr, Langhammer A. Serum 25-hydroxyvitamin D levels and self-reported allergic rhinitis in Norwegian adults: The HUNT Study. Allergy. 2014; 69:488–493.49. Maslova E, Hansen S, Jensen CB, Thorne-Lyman AL, Strøm M, Olsen SF. Vitamin D intake in mid-pregnancy and child allergic disease: a prospective study in 44,825 Danish mother-child pairs. BMC Pregnancy Childbirth. 2013; 13:199.
Article50. Cheng HM, Kim S, Park GH, Chang SE, Bang S, Won CH, Lee MW, Choi JH, Moon KC. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol. 2014; 133:1048–1055.
Article51. Toda M, Ono SJ. Genomics and proteomics of allergic disease. Immunology. 2002; 106:1–10.
Article52. Tizaoui K, Berraies A, Hamdi B, Kaabachi W, Hamzaoui K, Hamzaoui A. Association of vitamin D receptor gene polymorphisms with asthma risk: systematic review and updated meta-analysis of case-control studies. Lung. 2014; 192:955–965.
Article53. Tian HQ, Chen XY, Lu Y, Lu WM, Wang ML, Zhao HL, Lu MP, Zhou H, Chen RX, Zhang ZD, Shen C, Cheng L. Association of VDR and CYP2R1 Polymorphisms with Mite-Sensitized Persistent Allergic Rhinitis in a Chinese Population. PLoS One. 2015; 10:e0133162.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitamin D and Allergic Disease
- Vitamin D serum levels in allergic rhinitis: any difference from normal population?
- Upregulation of the Vitamin D Receptor in the Nasal Mucosa of Patients With Allergic Rhinitis
- The Role of Aviation Medical Examiners in the Diagnosis, Treatment and Aeromedical Assessment of Patients with Allergic Rhinitis
- Association between Vitamin D and Allergic Disease and Cataract in Korean Adults