Asia Pac Allergy.
2017 Jan;7(1):42-47. 10.5415/apallergy.2017.7.1.42.
The impact of age on Pru p 3 IgE production in Italy
- Affiliations
-
- 1Allergy and Laboratory Medicine Department, IRCCS-AOU San Martino-IST, 16132 Genoa, Italy. gio.cip@libero.it
- 2Department of Pediatrics, Foundation IRCCS Policlinico San Matteo, 27100 Pavia, Italy.
- 3Allergy Lab, Internal Medicine II Unit, AOU Cagliari, 09124 Cagliari, Italy.
- 4Immunohematology and Transfusion Medicine, AO G. Salvini, 20024, Garbagnate Milanese (MI), Italy.
- KMID: 2396913
- DOI: http://doi.org/10.5415/apallergy.2017.7.1.42
Abstract
- BACKGROUND
Pollen allergy may be frequently associated with fruit-vegetables: the so-called pollen food syndrome. Pru p 3 is the most relevant peach allergen. Previously, it has been reported that serum specific IgE level to Pru p 3 depends on age in a limited geographic area.
OBJECTIVE
This study aimed to to test the hypothesis about the differences of Pru p 3 sensitization across Italy, mainly concerning the impact of age.
METHODS
The current study was retrospective and multicentre, involving 2 labs in Northern Italy (709 subjects), 1 in Genoa (1,040 subjects), and 1 in Southern Italy (2,188 subjects). All of them referred to labs for IgE testing because of suspected food allergy. Serum IgE to Pru p 3 was assessed in all subjects.
RESULTS
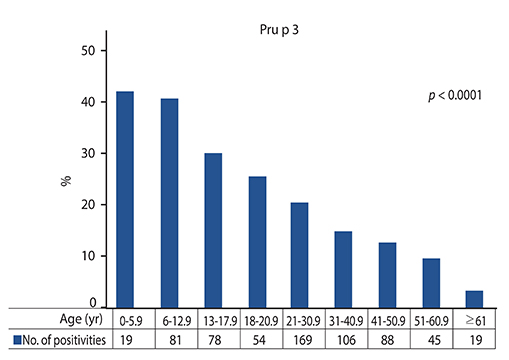
Sixteen point seven percent (16.7%) of subjects were sensitized to Pru p 3. Sensitization percentage sigificantly decreased over time. The serum IgE levels increased up to young adulthood and then decreased until aging.
CONCLUSION
Our experience demonstrates that Pru p 3 sensitization and production are closely age-dependent phenomena.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Asia Pacific allergy: 6 years old
Yoon-Seok Chang
Asia Pac Allergy. 2017;7(1):1-2. doi: 10.5415/apallergy.2017.7.1.1.
Reference
-
1. Pastorello EA, Robino AM. Clinical role of lipid transfer proteins in food allergy. Mol Nutr Food Res. 2004; 48:356–362.
Article2. Asero R, Antonicelli L, Arena A, Bommarito L, Caruso B, Crivellaro M, De Carli M, Della Torre E, Della Torre F, Heffler E, Lodi Rizzini F, Longo R, Manzotti G, Marcotulli M, Melchiorre A, Minale P, Morandi P, Moreni B, Moschella A, Murzilli F, Nebiolo F, Poppa M, Randazzo S, Rossi G, Senna GE. EpidemAAITO: features of food allergy in Italian adults attending allergy clinics: a multi-centre study. Clin Exp Allergy. 2009; 39:547–555.
Article3. Asero R, Mistrello G, Roncarolo D, Amato S, Caldironi G, Barocci F, van Ree R. Immunological cross-reactivity between lipid transfer proteins from botanically unrelated plant-derived foods: a clinical study. Allergy. 2002; 57:900–906.
Article4. Rossi RE, Monasterolo G, Canonica GW, Passalacqua G. Systemic reactions to peach are associated with high levels of specific IgE to Pru p 3. Allergy. 2009; 64:1795–1796.
Article5. Asero R, Arena A, Cecchi L, Conte ME, Crivellaro M, Emiliani F, Lodi Rizzini F, Longo R, Minale P, Murzilli F, Musarra A, Nebiolo F, Quercia O, Ridolo E, Savi E, Senna GE, Villalta D. Are IgE levels to foods other than rosaceae predictive of allergy in lipid transfer protein-hypersensitive patients? Int Arch Allergy Immunol. 2011; 155:149–154.
Article6. Murad A, Katelaris CH, Baumgart K. A case study of apple seed and grape allergy with sensitisation to nonspecific lipid transfer protein. Asia Pac Allergy. 2016; 6:129–132.
Article7. Leimgruber A, Mosimann B, Claeys M, Seppey M, Jaccard Y, Aubert V, Peitrequin R, Nisoli MP, Pécoud A. Clinical evaluation of a new in-vitro assay for specific IgE, the immuno CAP system. Clin Exp Allergy. 1991; 21:127–131.
Article8. Seagroatt V, Anderson SG. The second international reference preparation for human serum immunoglobulin E and the first British standard for human serum immunoglobulin E. J Biol Stand. 1981; 9:431–437.
Article9. Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010; 104:101–108.
Article10. Tuft L, Blumstein GI. Studies in food allergy: II. Sensitization to fresh fruits: clinical and experimental observations. J Allergy. 1942; 13:574–582.11. Price A, Ramachandran S, Smith GP, Stevenson ML, Pomeranz MK, Cohen DE. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015; 26:78–88.
Article12. Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, Knulst AC, Mari A, Muraro A, Ollert M, Poulsen LK, Vieths S, Worm M, Hoffmann-Sommergruber K. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. 2015; 70:1079–1090.
Article13. Ludman S, Jafari-Mamaghani M, Ebling R, Fox AT, Lack G, Du Toit G. Pollen food syndrome amongst children with seasonal allergic rhinitis attending allergy clinic. Pediatr Allergy Immunol. 2016; 27:134–140.
Article14. Mastrorilli C, Tripodi S, Caffarelli C, Perna S, Di Rienzo-Businco A, Sfika I, Asero R, Dondi A, Bianchi A, Povesi Dascola C, Ricci G, Cipriani F, Maiello N, Miraglia Del Giudice M, Frediani T, Frediani S, Macrì F, Pistoletti C, Dello Iacono I, Patria MF, Varin E, Peroni D, Comberiati P, Chini L, Moschese V, Lucarelli S, Bernardini R, Pingitore G, Pelosi U, Olcese R, Moretti M, Cirisano A, Faggian D, Travaglini A, Plebani M, Verga MC, Calvani M, Giordani P, Matricardi PM. Italian Pediatric Allergy Network (I-PAN). Endotypes of pollen-food syndrome in children with seasonal allergic rhinoconjunctivitis: a molecular classification. Allergy. 2016; 71:1181–1191.
Article15. Pascal M, Muñoz-Cano R, Reina Z, Palacín A, Vilella R, Picado C, Juan M, Sánchez-López J, Rueda M, Salcedo G, Valero A, Yagüe J, Bartra J. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012; 42:1529–1539.
Article16. Azofra J, Berroa F, Gastaminza G, Saiz N, Gamboa PM, Vela C, García BE, Lizarza S, Echenagusia MA, Joral A, Aranzabal MA, Quiñones MD, Jauregui I, Madera JF, Navarro JA, Lizaso MT, Bernad A, Goikoetxea MJ. Lipid transfer protein syndrome in a non-Mediterranean area. Int Arch Allergy Immunol. 2016; 169:181–188.
Article17. Fernández-Rivas M, González-Mancebo E, Rodríguez-Pérez R, Benito C, Sánchez-Monge R, Salcedo G, Alonso MD, Rosado A, Tejedor MA, Vila C, Casas ML. Clinically relevant peach allergy is related to peach lipid transfer protein, Pru p 3, in the Spanish population. J Allergy Clin Immunol. 2003; 112:789–795.18. Rodríguez-Perez R, Fernández-Rivas M, González-Mancebo E, Sánchez-Monge R, Díaz-Perales A, Salcedo G. Peach profilin: cloning, heterologous expression and cross-reactivity with Bet v 2. Allergy. 2003; 58:635–640.
Article19. Rodriguez J, Crespo JF, Lopez-Rubio A, De La Cruz-Bertolo J, Ferrando-Vivas P, Vives R, Daroca P. Clinical cross-reactivity among foods of the Rosaceae family. J Allergy Clin Immunol. 2000; 106(1 Pt 1):183–189.
Article20. García-Sellés FJ, Díaz-Perales A, Sánchez-Monge R, Alcántara M, Lombardero M, Barber D, Salcedo G, Fernández-Rivas M. Patterns of reactivity to lipid transfer proteins of plant foods and Artemisia pollen: an in vivo study. Int Arch Allergy Immunol. 2002; 128:115–122.21. Marknell Dewitt Å, Andersson K, Lidholm J. Cloning and sequence of the major peach allergen Pru p 1. Sequence accession No. ABB78006, direct submission. https://www.ncbi.nlm.nih.gov/protein/82492265/.22. Ahrazem O, Jimeno L, López-Torrejón G, Herrero M, Espada JL, Sánchez-Monge R, Duffort O, Barber D, Salcedo G. Assessing allergen levels in peach and nectarine cultivars. Ann Allergy Asthma Immunol. 2007; 99:42–47.
Article23. Fernández-Rivas M, Bolhaar S, González-Mancebo E, Asero R, van Leeuwen A, Bohle B, Ma Y, Ebner C, Rigby N, Sancho AI, Miles S, Zuidmeer L, Knulst A, Breiteneder H, Mills C, Hoffmann-Sommergruber K, van Ree R. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006; 118:481–488.
Article24. Asero R. Plant food allergies: a suggested approach to allergen-resolved diagnosis in the clinical practice by identifying easily available sensitization markers. Int Arch Allergy Immunol. 2005; 138:1–11.
Article25. Sánchez-Monge R, Lombardero M, García-Sellés FJ, Barber D, Salcedo G. Lipid-transfer proteins are relevant allergens in fruit allergy. J Allergy Clin Immunol. 1999; 103(3 Pt 1):514–519.
Article26. Asero R, Amato S, Alfieri B, Folloni S, Mistrello G. Rice: another potential cause of food allergy in patients sensitized to lipid transfer protein. Int Arch Allergy Immunol. 2007; 143:69–74.
Article27. Carnés J, Fernández-Caldas E, Gallego MT, Ferrer A, Cuesta-Herranz J. Pru p 3 (LTP) content in peach extracts. Allergy. 2002; 57:1071–1075.
Article28. Brenna OV, Pastorello EA, Farioli L, Pravettoni V, Pompei C. Presence of allergenic proteins in different peach (Prunus persica) cultivars and dependence of their content on fruit ripening. J Agric Food Chem. 2004; 52:7997–8000.
Article29. Sánchez-Monge R, Blanco C, López-Torrejón G, Cumplido J, Recas M, Figueroa J, Carrillo T, Salcedo G. Differential allergen sensitization patterns in chestnut allergy with or without associated latex-fruit syndrome. J Allergy Clin Immunol. 2006; 118:705–710.30. Palacín A, Cumplido J, Figueroa J, Ahrazem O, Sánchez-Monge R, Carrillo T, Salcedo G, Blanco C. Cabbage lipid transfer protein Bra o 3 is a major allergen responsible for cross-reactivity between plant foods and pollens. J Allergy Clin Immunol. 2006; 117:1423–1429.31. Pastorello EA, Farioli L, Pravettoni V, Ortolani C, Fortunato D, Giuffrida MG, Perono Garoffo L, Calamari AM, Brenna O, Conti A. Identification of grape and wine allergens as an endochitinase 4, a lipid-transfer protein, and a thaumatin. J Allergy Clin Immunol. 2003; 111:350–359.
Article32. Asero R, Mistrello G, Roncarolo D, de Vries SC, Gautier MF, Ciurana CL, Verbeek E, Mohammadi T, Knul-Brettlova V, Akkerdaas JH, Bulder I, Aalberse RC, van Ree R. Lipid transfer protein: a pan-allergen in plant-derived foods that is highly resistant to pepsin digestion. Int Arch Allergy Immunol. 2000; 122:20–32.
Article33. Asero R, Mistrello G, Amato S, Roncarolo D, Martinelli A, Zaccarini M. Peach fuzz contains large amounts of lipid transfer protein: is this the cause of the high prevalence of sensitization to LTP in Mediterranean countries? Eur Ann Allergy Clin Immunol. 2006; 38:118–121.34. Willerroider M, Fuchs H, Ballmer-Weber BK, Focke M, Susani M, Thalhamer J, Ferreira F, Wüthrich B, Scheiner O, Breiteneder H, Hoffmann-Sommergruber K. Cloning and molecular and immunological characterisation of two new food allergens, Cap a 2 and Lyc e 1, profilins from bell pepper (Capsicum annuum) and Tomato (Lycopersicon esculentum). Int Arch Allergy Immunol. 2003; 131:245–255.35. Thorn KS, Christensen HE, Shigeta R, Huddler D, Shalaby L, Lindberg U, Chua NH, Schutt CE. The crystal structure of a major allergen from plants. Structure. 1997; 5:19–32.
Article36. Tosca MA, Silvestri M, Olcese R, Sacco O, Pistorio A, Rossi GA, Ciprandi G. Allergen-specific IgE to food molecular components and age: from early childhood to adulthood. Allergol Immunopathol (Madr). 2017; 45:87–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Age Impact on Serum Total and Allergen-Specific IgE
- Local Production of IgE in Nasal Polyp
- Mugwort Pollen-Related Food Allergy: Lipid Transfer Protein Sensitization and Correlation With the Severity of Allergic Reactions in a Chinese Population
- IgE Level in Atopic Dermatitis
- The effect of interleukin 4 and hydrocortisone on the synthesis of IgE antibodies by peripheral mononuclear cells from atopic patients