Asia Pac Allergy.
2017 Jan;7(1):10-18. 10.5415/apallergy.2017.7.1.10.
Pranlukast reduces asthma exacerbations during autumn especially in 1- to 5-year-old boys
- Affiliations
-
- 1Department of Pediatrics, Graduate School of Medicine, Chiba University, Chiba 263-8522, Japan. m0ritan@hotmail.com
- 2Department of Pediatrics, Chiba Kaihin Municipal Hospital, Chiba 261-0012, Japan.
- 3Department of Pediatrics, Shimoshizu National Hospital, Chiba 284-0003, Japan.
- 4Satou Clinic, Arao 864-0041, Japan.
- 5Department of Allergy and Rheumatology, Chiba Children's Hospital, Chiba 266-0007, Japan.
- 6Abe Hiroki Children's Clinic, Chiba 264-0028, Japan.
- 7Sunrise Children's Clinic, Chiba 273-0035, Japan.
- 8Tsubaki Children's Clinic, Chiba 260-0001, Japan.
- 9Mana Children's Clinic, Chiba 266-0032, Japan.
- 10Katsuyama Clinic, Chiba 299-2117, Japan.
- 11Nakayama Clinic, Chiba 275-0021, Japan.
- 12Higashi Koiwa Wanpaku Clinic, Tokyo 133-0052, Japan.
- 13Department of Pediatrics, National Hospital Organization Chiba Medical Center, Chiba 260-8606, Japan.
- 14Chiba Rosai Hospital, Ichihara 290-0003, Japan.
- KMID: 2396909
- DOI: http://doi.org/10.5415/apallergy.2017.7.1.10
Abstract
- BACKGROUND
Leukotriene receptor antagonists have been used to prevent virus-induced asthma exacerbations in autumn. Its efficacy, however, might differ with age and sex.
OBJECTIVE
This study aimed to investigate whether pranlukast added to usual asthma therapy in Japanese children during autumn, season associated with the peak of asthma, reduces asthma exacerbations. It was also evaluated the effect of age and sex on pranlukast's efficacy.
METHODS
A total of 121 asthmatic children aged 1 to 14 years were randomly assigned to receive regular pranlukast or not according to sex, and were divided in 2 age groups, 1-5 years and 6-14 years. The primary outcome was total asthma score calculated during 8 weeks by using a sticker calendar related to the days in which a child experienced a worsening of asthma symptoms. This open study lasted 60 days from September 15 to November 14, 2007.
RESULTS
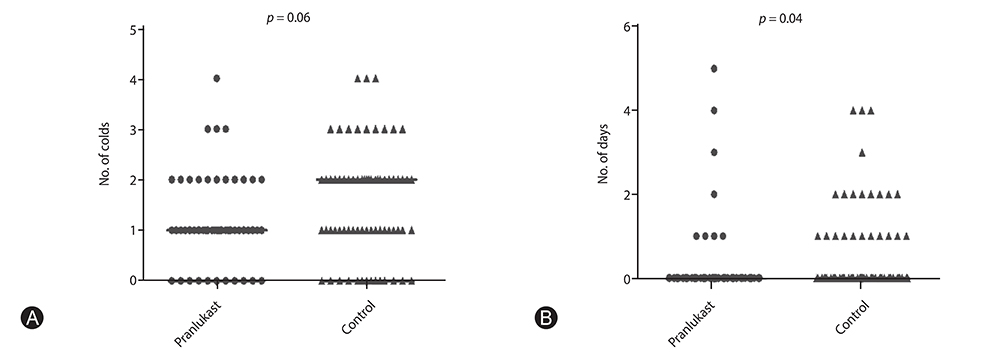
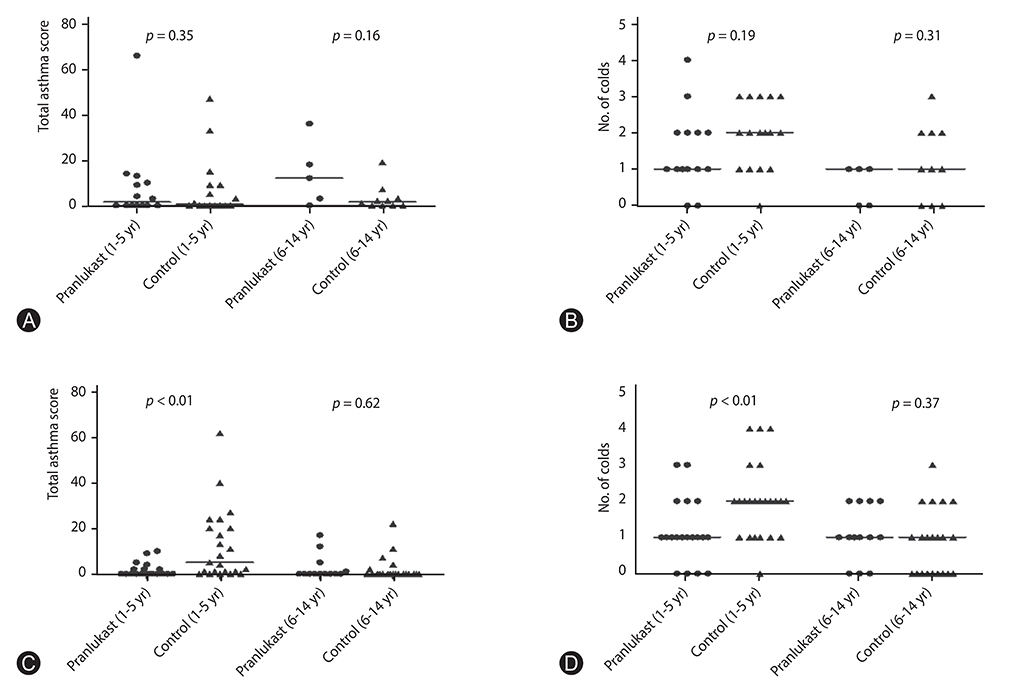
Significant differences in pranlukast efficacy were observed between sex and age groups. Boys aged 1 to 5 years had the lower total asthma score at 8 weeks (p = 0.002), and experienced fewer cold episodes (p = 0.007). There were no significant differences between pranlukast and control group in total asthma score at 8 weeks (p = 0.35), and in the days in which a child experienced a worsening of asthma symptoms (p = 0.67).
CONCLUSION
There was a substantial benefit of adding pranlukast to usual therapy in asthmatic children, especially in boys aged 1 to 5 years, during autumn season.
Keyword
- Age; Asthma; Child; Exacerbations; Sex; Pranlukast
MeSH Terms
Figure
Cited by 1 articles
-
Asia Pacific allergy: 6 years old
Yoon-Seok Chang
Asia Pac Allergy. 2017;7(1):1-2. doi: 10.5415/apallergy.2017.7.1.1.
Reference
-
1. Korhonen K, Reijonen TM, Remes K, Malmström K, Klaukka T, Korppi M. Reasons for and costs of hospitalization for pediatric asthma: a prospective 1-year follow-up in a population-based setting. Pediatr Allergy Immunol. 2001; 12:331–338.
Article2. Weinberger M. Treatment strategies for viral respiratory infection-induced asthma. J Pediatr. 2003; 142:2 Suppl. S34–S38.
Article3. Caramori G, Ito K, Contoli M, Di Stefano A, Johnston SL, Adcock IM, Papi A. Molecular mechanisms of respiratory virus-induced asthma and COPD exacerbations and pneumonia. Curr Med Chem. 2006; 13:2267–2290.4. Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011; 128:1165–1174.
Article5. Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006; 61:722–728.6. Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, Tozzi CA, Polos P. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005; 171:315–322.
Article7. Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, Sears MR. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007; 120:e702–e712.
Article8. Robertson CF, Price D, Henry R, Mellis C, Glasgow N, Fitzgerald D, Lee AJ, Turner J, Sant M. Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am J Respir Crit Care Med. 2007; 175:323–329.9. Almqvist C, Worm M, Leynaert B. working group of GA2LEN WP 2.5 Sex. Impact of sex on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008; 63:47–57.10. Morikawa A, Nishima S. Japanese Society of Pediatric Allergy and Clinical Immunology. New Japanese pediatric guidelines for the treatment and management of bronchial asthma. Pediatr Int. 2007; 49:1023–1031.
Article11. Asano K, Shiomi T, Hasegawa N, Nakamura H, Kudo H, Matsuzaki T, Hakuno H, Fukunaga K, Suzuki Y, Kanazawa M, Yamaguchi K. Leukotriene C4 synthase gene A(-444)C polymorphism and clinical response to a CYS-LT(1) antagonist, pranlukast, in Japanese patients with moderate asthma. Pharmacogenetics. 2002; 12:565–570.
Article12. Telleria JJ, Blanco-Quiros A, Varillas D, Armentia A, Fernandez-Carvajal I, Jesus Alonso M, Diez I. ALOX5 promoter genotype and response to montelukast in moderate persistent asthma. Respir Med. 2008; 102:857–861.
Article13. Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014; 209:Suppl 3. S120–S126.
Article14. Kozer E, Lotem Z, Elgarushe M, Torgovicky R, Cohen R, Cohen HA, Berkovitch M. RCT of montelukast as prophylaxis for upper respiratory tract infections in children. Pediatrics. 2012; 129:e285–e290.
Article15. Horiguchi T, Ohira D, Kobayashi K, Hirose M, Miyazaki J, Kondo R, Tachikawa S. Clinical evaluation of leukotriene receptor antagonists in preventing common cold-like symptoms in bronchial asthma patients. Allergol Int. 2007; 56:263–267.
Article16. Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF Jr. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008; 178:667–672.
Article17. Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL;. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007; 151:34–42. 42.e1
Article18. Gentile DA, Fireman P, Skoner DP. Elevations of local leukotriene C4 levels during viral upper respiratory tract infections. Ann Allergy Asthma Immunol. 2003; 91:270–274.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pranlukast-Induced Anaphylactic Shock
- New Tread of Association of Rhinovirus and Asthma
- Seasonality of asthma exacerbation in children caused by respiratory virus infection and allergen sensitization
- Current Control and Future Risk in Asthma Management
- Treatment of mild asthma: Is it necessary to keep regular inhaled corticosteroids?