Int J Thyroidol.
2017 Nov;10(2):127-132. 10.11106/ijt.2017.10.2.127.
Anaplastic Transformation of Follicular Thyroid Cancer in the Lung, Liver, Bone, and Adrenal Gland
- Affiliations
-
- 1Department of Pathology, Dankook University College of Medicine, Cheonan, Korea.
- 2Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea. dh9070@dankook.ac.kr
- 3Department of Kinesiologic Medical Science, Graduate, Dankook University, Cheonan, Korea.
- KMID: 2396819
- DOI: http://doi.org/10.11106/ijt.2017.10.2.127
Abstract
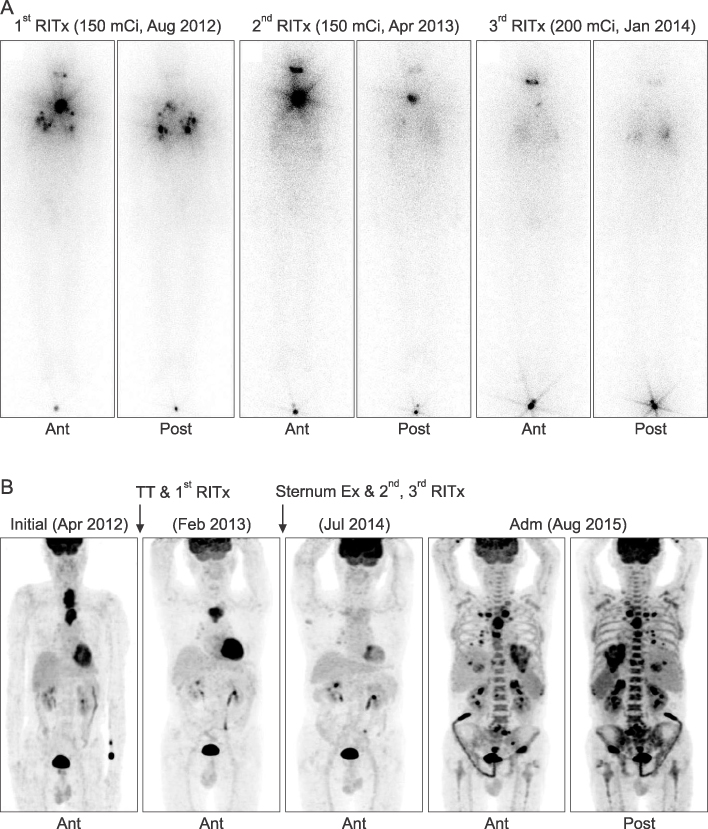
- Anaplastic transformation of differentiated thyroid cancer at distant metastatic sites is extremely rare and has a poor prognosis. It usually occurs in the thyroid gland or cervical lymph nodes. Here we report a case of anaplastic transformation arising at multiple distant metastatic sites including the lung, liver, adrenal gland, bone, and lymph nodes in a patient 3 years after total thyroidectomy for follicular thyroid cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012; 22(11):1104–1139.
Article2. Wiseman SM, Loree TR, Rigual NR, Hicks WL Jr, Douglas WG, Anderson GR, et al. Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck. 2003; 25(8):662–670.
Article3. Nakayama R, Horiuchi K, Susa M, Hosaka S, Hayashi Y, Kameyama K, et al. Anaplastic transformation of follicular thyroid carcinoma in a metastatic skeletal lesion presenting with paraneoplastic leukocytosis. Thyroid. 2012; 22(2):200–204.
Article4. Solomon JP, Wen F, Jih LJ. Anaplastic transformation of papillary thyroid cancer in the retroperitoneum. Case Rep Pathol. 2015; 2015:241308.
Article5. Abe T, Suzuki M, Shimizu K, Shinagawa N, Oizumi S, Matsuno Y, et al. Anaplastic transformation of papillary thyroid carcinoma in multiple lung metastases presenting with a malignant pleural effusion: a case report. J Med Case Rep. 2014; 8:460.
Article6. Al-Qsous W, Miller ID. Anaplastic transformation in lung metastases of differentiated papillary thyroid carcinoma: an autopsy case report and review of the literature. Ann Diagn Pathol. 2010; 14(1):41–43.
Article7. Sotome K, Onishi T, Hirano A, Nakamaru M, Furukawa A, Miyazaki H, et al. A rare case of anaplastic transformation within the metastatic site of the retroperitoneal region in a patient 17 years after total thyroidectomy for papillary carcinoma of the thyroid beginning with multiple bone metastases. Thyroid. 2007; 17(12):1309–1311.
Article8. Moore JH Jr, Bacharach B, Choi HY. Anaplastic transformation of metastatic follicular carcinoma of the thyroid. J Surg Oncol. 1985; 29(4):216–221.
Article9. Khairy G. Anaplastic transformation of differentiated thyroid carcinoma. Int J Health Sci (Qassim). 2009; 3(1):93–96.10. Benedict M, Costa J. Metastatic papillary thyroid carcinoma with multifocal synchronous transformation to anaplastic thyroid carcinoma. Case Rep Pathol. 2016; 2016:4863405.
Article11. Kim JS, Moon HJ, Han JS, Kim MJ. Importance of regular follow-up examination during active surveillance: a case of anaplastic transformation of papillary thyroid microcarcinoma. Int J Thyroidol. 2016; 9(2):185–189.
Article12. Ambelil M, Sultana S, Roy S, Gonzalez MM. Anaplastic transformation in mandibular metastases of follicular variant of papillary thyroid carcinoma: a case report and review of the literature. Ann Clin Lab Sci. 2016; 46(5):552–556.13. Sumida T, Hamakawa H, Imaoka M, Okamoto N, Takarada M, Tanioka H, et al. A case of submandibular malignant rhabdoid tumor transformed from papillary thyroid carcinoma. J Oral Pathol Med. 2001; 30(7):443–447.
Article14. Takeshita Y, Takamura T, Minato H, Misu H, Ando H, Yamashita T, et al. Transformation of p53-positive papillary thyroid carcinoma to anaplastic carcinoma of the liver following postoperative radioactive iodine-131 therapy. Intern Med. 2008; 47(19):1709–1712.
Article15. Angeles-Angeles A, Chable-Montero F, Martinez-Benitez B, Albores-Saavedra J. Unusual metastases of papillary thyroid carcinoma: report of 2 cases. Ann Diagn Pathol. 2009; 13(3):189–196.
Article16. Kaushal S, Sharma MC, Mathur SR, Rastogi S, Bal CS, Chumber S. Anaplastic transformation of metastatic papillary thyroid carcinoma at shoulder mimicking soft tissue sarcoma. Indian J Pathol Microbiol. 2011; 54(4):796–799.17. Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid. 2013; 23(6):709–713.
Article18. Xu B, Ghossein R. Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocr Pathol. 2016; 27(3):205–212.
Article19. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016; 126(3):1052–1066.
Article20. Sera N, Ashizawa K, Ando T, Ide A, Abe Y, Usa T, et al. Anaplastic changes associated with p53 gene mutation in differentiated thyroid carcinoma after insufficient radioactive iodine (131I) therapy. Thyroid. 2000; 10(11):975–979.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SPARC Expression in Thyroid Follicular Adenomas and Carcinomas
- Importance of Regular Follow-Up Examination during Active Surveillance: a Case of Anaplastic Transformation of Papillary Thyroid Microcarcinoma
- An Unusual Case of Metastatic Non-Small Cell Lung Cancer Misidentified as Anaplastic Thyroid Cancer
- A Follicular Thyroid Carcinoma Presenting as Single Bone Metastasis to Distal Femur with Pathologic Fracture: a Case Report
- A Case of Thyroid Anaplastic Cancer with Intestinal Metastasis