Clin Exp Otorhinolaryngol.
2017 Dec;10(4):349-356. 10.21053/ceo.2016.00941.
The Efficacy of Fibroblast Growth Factor for the Treatment of Chronic Vocal Fold Scarring: From Animal Model to Clinical Application
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 2Department of Otolaryngology-Head and Neck Surgery, Soonchunhyang University College of Medicine, Seoul, Korea.
- 3Department of Otolaryngology-Head and Neck Surgery, Soonchunhyang University College of Medicine, Bucheon, Korea. lsw0922@schmc.ac.kr
- 4Department of Pathology, Soonchunhyang University College of Medicine, Bucheon, Korea.
- KMID: 2396791
- DOI: http://doi.org/10.21053/ceo.2016.00941
Abstract
OBJECTIVES
This study assessed the regenerative efficacy of basic fibroblast growth factor (FGF) in a rabbit model of chronic vocal fold scarring and then confirmed its utility and safety in a prospective trial of patients with this condition.
METHODS
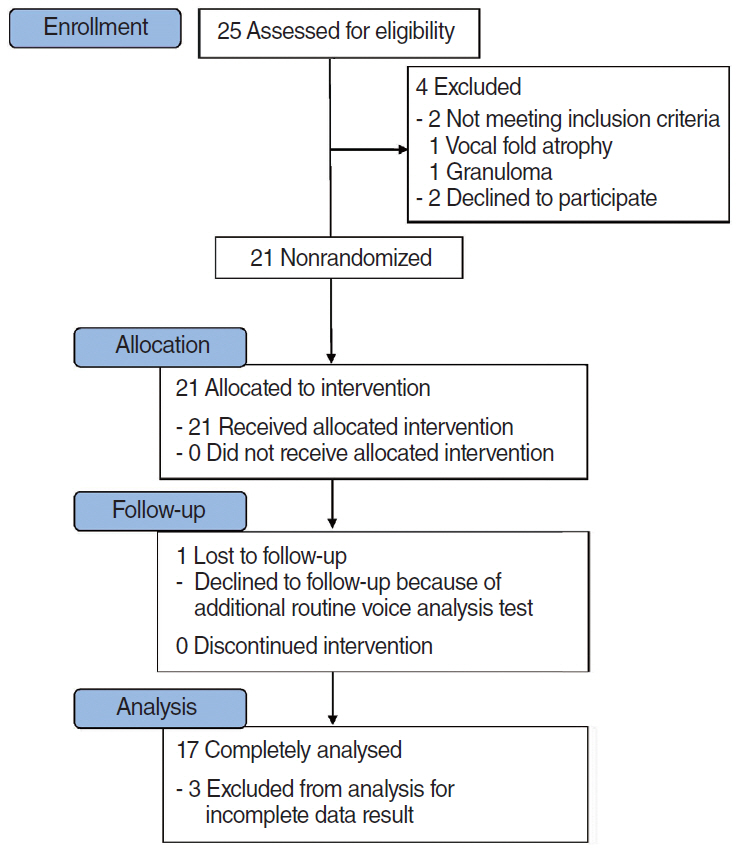
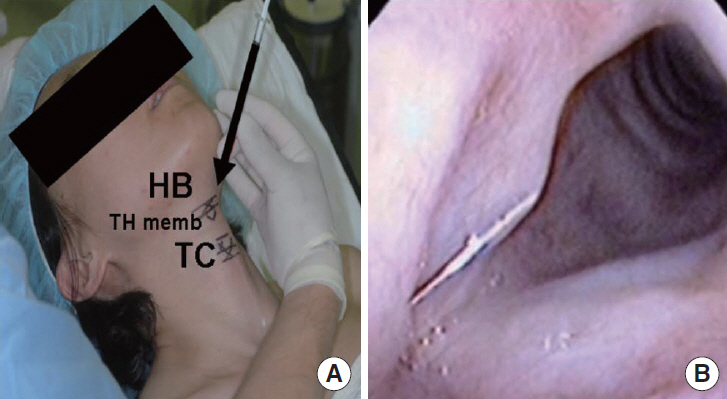
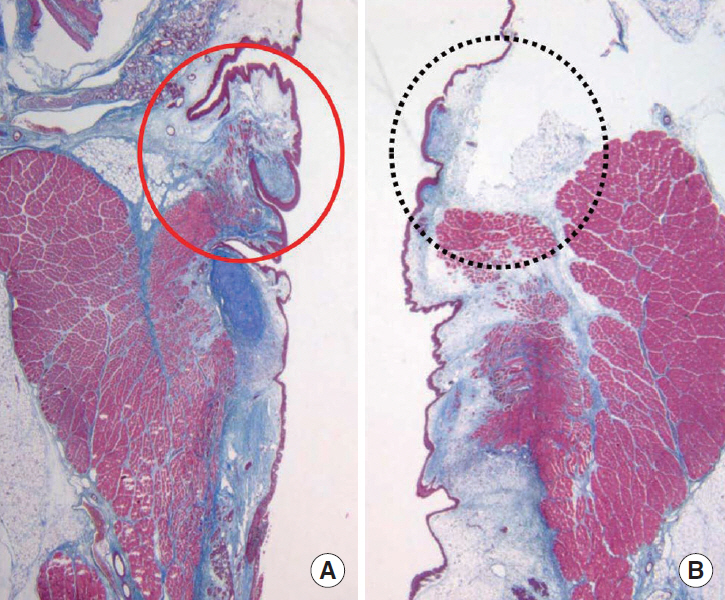
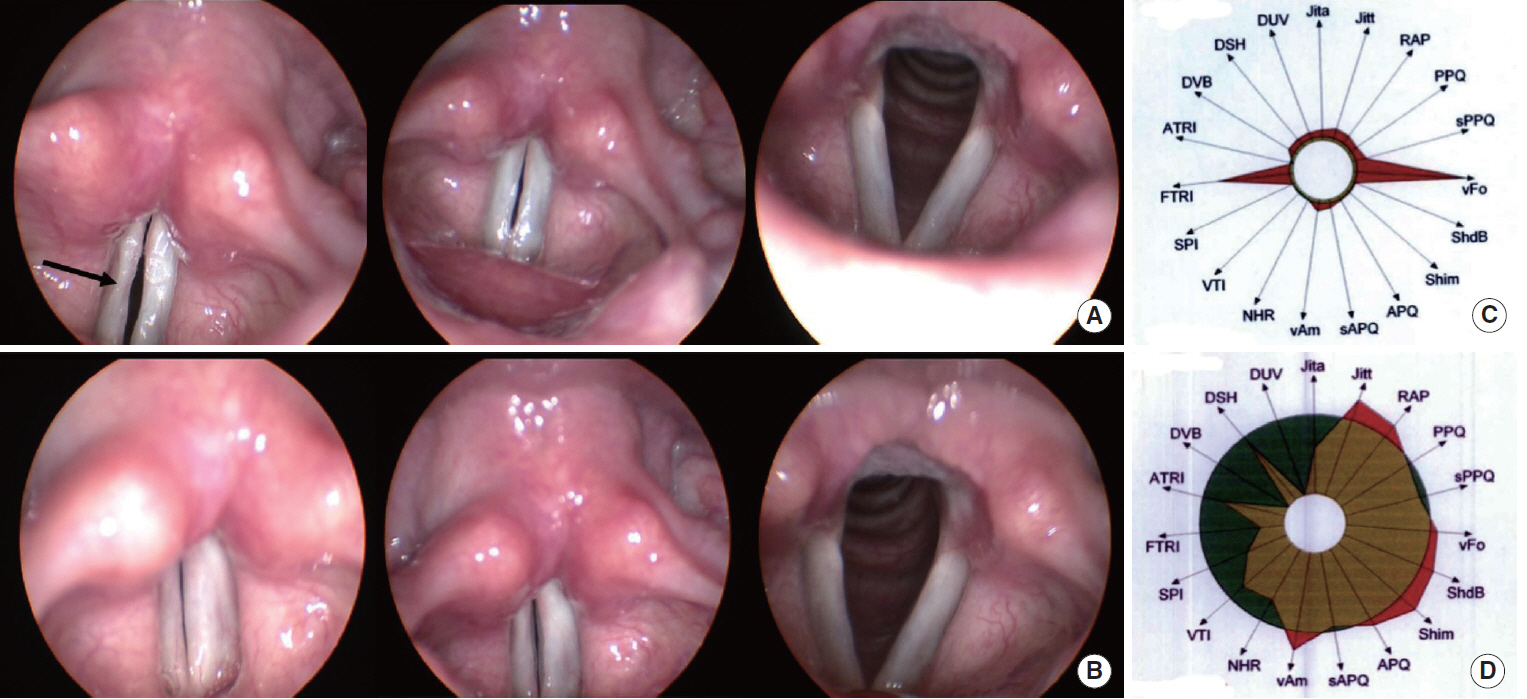
FGF was injected three times, at 1-week intervals, into a chronic vocal fold scar created in a rabbit model. After 1 month, mRNA level of procollagen I, hyaluronic acid synthetase 2 (HAS 2), and matrix metalloproteinase 2 (MMP 2) were analyzed by real-time polymerase chain reaction. The relative densities of hyaluronic acid (HA) and collagen were examined 3 months post-injection. From April 2012 to September 2014, a prospective clinical trial was conducted at a tertiary hospital in Korea. FGF was injected into the mild vocal fold scar of 17 consecutive patients with a small glottic gap. The patients underwent perceptual, stroboscopic, acoustic aerodynamic test, and Voice Handicap Index (VHI) survey prior to and 3, 6, and 12 months after FGF injection.
RESULTS
FGF injection of the vocal fold scar decreased the density of collagen and increased mRNA level of HAS 2 and MMP 2 expression significantly compared to the control group injected with phosphate buffered solution in a rabbit model (P < 0.05). In the clinical trial, significant improvements in the majority of the subjective and objective voice parameters were registered 3 months after FGF injection and were maintained at 12 months. Complications associated with the FGF injections, such as granuloma, were not observed during the follow-up period.
CONCLUSION
Based on the animal model and the prospective clinical trial, vocal fold injections of FGF in patients with mild chronic vocal fold scarring can significantly improve voice quality for as long as 1 year and without side effects. Our results recommend the use of FGF vocal fold injection as an alternative treatment modality for mild chronic vocal fold scarring.
Keyword
MeSH Terms
-
Acoustics
Animals*
Cicatrix*
Collagen
Dysphonia
Fibroblast Growth Factor 2
Fibroblast Growth Factors*
Fibroblasts*
Follow-Up Studies
Granuloma
Humans
Hyaluronic Acid
Korea
Ligases
Matrix Metalloproteinase 2
Models, Animal*
Procollagen
Prospective Studies
Real-Time Polymerase Chain Reaction
RNA, Messenger
Specific Gravity
Tertiary Care Centers
Vocal Cords*
Voice
Voice Quality
Collagen
Fibroblast Growth Factor 2
Fibroblast Growth Factors
Hyaluronic Acid
Ligases
Matrix Metalloproteinase 2
Procollagen
RNA, Messenger
Figure
Reference
-
1. Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005; Jun. 13(3):143–7.
Article2. Suehiro A, Hirano S, Kishimoto Y, Rousseau B, Nakamura T, Ito J. Treatment of acute vocal fold scar with local injection of basic fibroblast growth factor: a canine study. Acta Otolaryngol. 2010; Jul. 130(7):844–50.
Article3. Rousseau B, Hirano S, Chan RW, Welham NV, Thibeault SL, Ford CN, et al. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004; Mar. 18(1):116–24.
Article4. Lim JY, Choi BH, Lee S, Jang YH, Choi JS, Kim YM. Regulation of wound healing by granulocyte-macrophage colony-stimulating factor after vocal fold injury. PLoS One. 2013; 8(1):e54256.
Article5. Lee SW, Kim JW, Lee JY, Chang HS, Kim HK, Choi EC. Augmentation of vocal fold using a fat block implant following cordotomy through a minithyrotomy approach in a rabbit model. J Voice. 2012; Jul. 26(4):521–5.
Article6. Hirano M. Clinical examination of voice. Wien: Springer-Verlag;1981.7. Yun YS, Kim HH, Son YI, Choi HS. Validation of the Korean Voice Handicap Index (K-VHI) and the clinical usefulness of Korean VHI-10. Korean J Commun Disord. 2008; Jun. 13(2):216–41.8. Lee SW, Kim JW, Koh YW, Shim SS, Son YI. Comparative analysis of efficiency of injection laryngoplasty technique for with or without neck treatment patients: a transcartilaginous approach versus the cricothyroid approach. Clin Exp Otorhinolaryngol. 2010; Mar. 3(1):37–41.
Article9. Yoon KC, Mun GH, Kim SD, Kim SH, Kim CY, Park KH, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; Dec. 25(6):421–33.
Article10. Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009; Jul. 23(4):399–407.
Article11. Dailey SH, Ford CN. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol Clin North Am. 2006; Feb. 39(1):23–42.
Article12. Kanemaru S, Nakamura T, Omori K, Kojima H, Magrufov A, Hiratsuka Y, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003; Nov. 112(11):915–20.
Article13. Hirano S, Bless D, Heisey D, Ford C. Roles of hepatocyte growth factor and transforming growth factor beta1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope. 2003; Jan. 113(1):144–8.14. Hirano S, Bless DM, Heisey D, Ford CN. Effect of growth factors on hyaluronan production by canine vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2003; Jul. 112(7):617–24.
Article15. Hirano S, Bless DM, Rousseau B, Welham N, Montequin D, Chan RW, et al. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004; Mar. 114(3):548–56.
Article16. Hirano S, Bless DM, Nagai H, Rousseau B, Welham NV, Montequin DW, et al. Growth factor therapy for vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2004; Oct. 113(10):777–85.
Article17. Ohno T, Hirano S, Kanemaru S, Yamashita M, Umeda H, Suehiro A, et al. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2007; Oct. 116(10):762–9.
Article18. Hirano S, Mizuta M, Kaneko M, Tateya I, Kanemaru SI, Ito J. Regenerative phonosurgical treatments for vocal fold scar and sulcus with basic fibroblast growth factor. Laryngoscope. 2013; Nov. 123(11):2749–55.
Article19. Hong HH, Trackman PC. Cytokine regulation of gingival fibroblast lysyl oxidase, collagen, and elastin. J Periodontol. 2002; Feb. 73(2):145–52.
Article20. Heldin P, Laurent TC, Heldin CH. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem J. 1989; Mar. 258(3):919–22.
Article21. Kanazawa T, Komazawa D, Indo K, Akagi Y, Lee Y, Nakamura K, et al. Single injection of basic fibroblast growth factor to treat severe vocal fold lesions and vocal fold paralysis. Laryngoscope. 2015; Oct. 125(1):E338–44.
Article22. Tateya T, Tateya I, Bless DM. Immuno-scanning electron microscopy of collagen types I and III in human vocal fold lamina propria. Ann Otol Rhinol Laryngol. 2007; Feb. 116(2):156–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histological Effect of Basic Fibroblast Growth Factor on Chronic Vocal Fold Scarring in a Rat Model
- A Case of Surgical Treatment of Intractable Vocal Fold Scar Using Basic Fibroblast Growth Factor and Collagen Scaffold
- Vocal Fold Injection: Review of Indications, Techniques, and Materials for Augmentation
- Vocal Fold Regeneration: Current Review
- The Study of Bilateral Venign Vocal Fold Lesions