Korean Circ J.
2017 Nov;47(6):907-917. 10.4070/kcj.2017.0108.
Plaque Characteristics and Ruptured Plaque Location according to Lesion Geometry in Culprit Lesions of ST-Segment Elevation Myocardial Infarction
- Affiliations
-
- 1Department of Cardiology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. sesim1989@gmail.com
- 2Department of Internal Medicine and Cardiovascular Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Cardiology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 4Department of Cardiology, Inje University Ilsan Paik Hospital, Goyang, Korea.
- 5Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 6Suntech Research Center, Seoul, Korea.
- 7Department of Internal Medicine and Cardiovascular Center, Seoul National University Hospital, Seoul, Korea.
- 8Institute of Aging, Seoul National University, Seoul, Korea.
- KMID: 2396484
- DOI: http://doi.org/10.4070/kcj.2017.0108
Abstract
- BACKGROUND AND OBJECTIVES
The correlations between plaque characteristics and plaque rupture location according to segmental lesion analysis have not been well defined. The aim of this study was to assess those characteristics of ST-segment elevation myocardial infarction (STEMI) culprit lesions according to segmental lesion geometry using virtual histology intravascular ultrasound (VH-IVUS).
METHODS
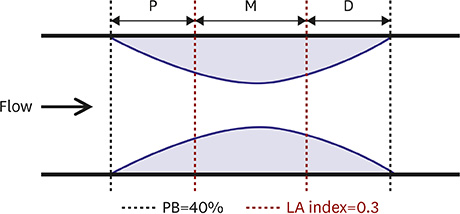
Sixty single discrete lesions found in the left anterior descending (LAD) coronary arteries of 60 patients with STEMI were included. Each lesion was divided into 3 segments based on lumen area (LA) index, calculated by dividing the lesion LA by the reference LA.
RESULTS
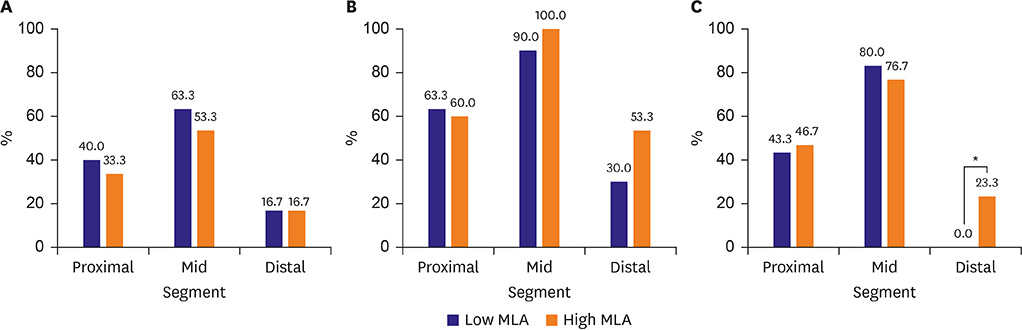
Among the 3 segments, the mid-segment showed the highest proportion of necrotic core (NC; proximal, mid-, and distal segments: 20.9±11.8%, 22.7±11.3%, and 17.5±11.2%, respectively, p=0.044). VH-IVUS-derived thin-cap fibroatheroma (VH-TCFA) was also more frequently found in the mid-segment than in proximal and distal segments (36.7%, 58.3%, and 16.7%, p < 0.001). The mid-segment also showed the highest prevalence of plaque rupture (45.0%, 78.3% and 11.7%, p < 0.001) and thrombus (61.7%, 95.0%, and 41.7%, p < 0.001) compared to proximal or distal segments. When the lesions were divided into 2 groups according to the median value (4.0 mm2) of minimum lumen area (MLA), plaque rupture at the distal segment was observed only in high MLA lesions (23.3% vs. 0.0%, p=0.011).
CONCLUSION
Analysis of longitudinal lesion geometry using the LA index can be useful in evaluating plaque vulnerability and the incidence of plaque rupture and thrombus in STEMI patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Dong L, Mintz GS, Witzenbichler B, et al. Comparison of plaque characteristics in narrowings with ST-elevation myocardial infarction (STEMI), non-STEMI/unstable angina pectoris and stable coronary artery disease (from the ADAPT-DES IVUS Substudy). Am J Cardiol. 2015; 115:860–866.2. Jang HJ, Koo BK, Lee HS, et al. Safety and efficacy of a novel hyperaemic agent, intracoronary nicorandil, for invasive physiological assessments in the cardiac catheterization laboratory. Eur Heart J. 2013; 34:2055–2062.3. Takaoka N, Tsujita K, Kaikita K, et al. Comprehensive analysis of intravascular ultrasound and angiographic morphology of culprit lesions between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome. Int J Cardiol. 2014; 171:423–430.4. Maehara A, Mintz GS, Bui AB, et al. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J Am Coll Cardiol. 2002; 40:904–910.5. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011; 364:226–235.6. Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011; 124:779–788.7. Park JB, Choi G, Chun EJ, et al. Computational fluid dynamic measures of wall shear stress are related to coronary lesion characteristics. Heart. 2016; 102:1655–1661.8. Lee JM, Choi G, Hwang D, et al. Impact of longitudinal lesion geometry on location of plaque rupture and clinical presentations. JACC Cardiovasc Imaging. 2017; 10:677–688.9. American College of Emergency Physicians. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61:e78–e140.10. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus). A report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2001; 37:1478–1492.11. García-García HM, Mintz GS, Lerman A, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009; 5:177–189.12. Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003; 108:1664–1672.13. Niccoli G, Montone RA, Di Vito L, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015; 36:1377–1384.14. Kwak BR, Bäck M, Bochaton-Piallat ML, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014; 35:3013–3020. 3020a–3020d.15. Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol. 2008; 51:645–650.16. Lee KE, Kim GT, Lee JS, Chung JH, Shin ES, Shim EB. A patient-specific virtual stenotic model of the coronary artery to analyze the relationship between fractional flow reserve and wall shear stress. Int J Cardiol. 2016; 222:799–805.17. Tanaka A, Shimada K, Namba M, et al. Relationship between longitudinal morphology of ruptured plaques and TIMI flow grade in acute coronary syndrome: a three-dimensional intravascular ultrasound imaging study. Eur Heart J. 2008; 29:38–44.18. Slager CJ, Wentzel JJ, Gijsen FJ, et al. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat Clin Pract Cardiovasc Med. 2005; 2:456–464.19. Choi G, Lee JM, Kim HJ, et al. Coronary artery axial plaque stress and its relationship with lesion geometry: application of computational fluid dynamics to coronary CT angiography. JACC Cardiovasc Imaging. 2015; 8:1156–1166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute coronary syndrome and vulnerable plaque
- ST Segment Depression in Lateral Leads in Inferior Wall Acute Myocardial Infarction
- Multimodality Intravascular Imaging Assessment of Plaque Erosion versus Plaque Rupture in Patients with Acute Coronary Syndrome
- Accuracy of the Electrocardiographic Criteria for Predicting the Right or Left Circumflex Coronary Artery as the Culprit Coronary Artery in Acute Inferior Myocardial Infarction
- Relationship between ST segment of lateral leads and culprit arteries in acute inferior myocardial infarction