Korean Circ J.
2017 Nov;47(6):842-857. 10.4070/kcj.2017.0105.
Where Is the “Optimal†Fontan Hemodynamics?
- Affiliations
-
- 1Departments of Pediatric Cardiology and Adult Congenital Heart Disease, National Cerebral and Cardiovascular Center, Suita, Japan. hohuchi@hsp.ncvc.go.jp
- KMID: 2396476
- DOI: http://doi.org/10.4070/kcj.2017.0105
Abstract
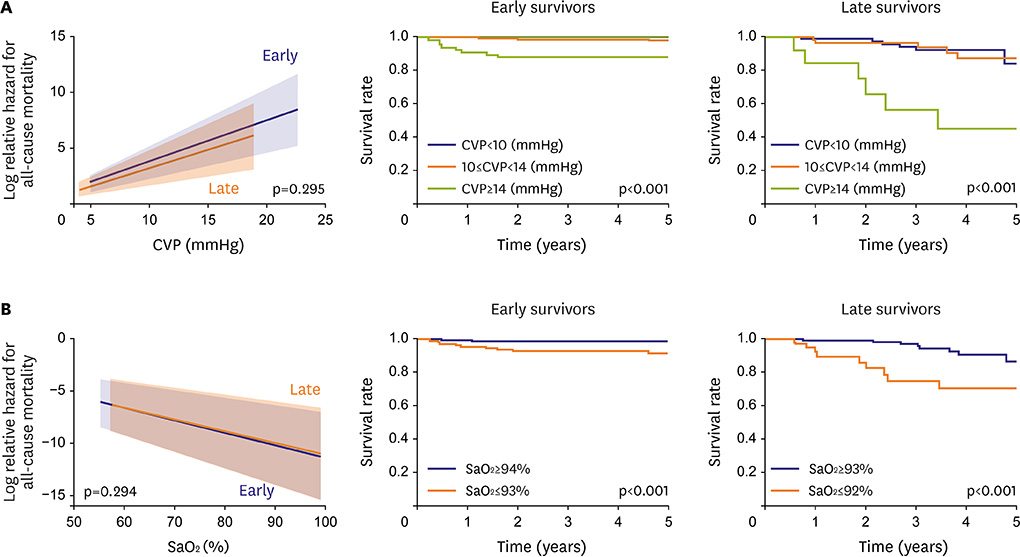
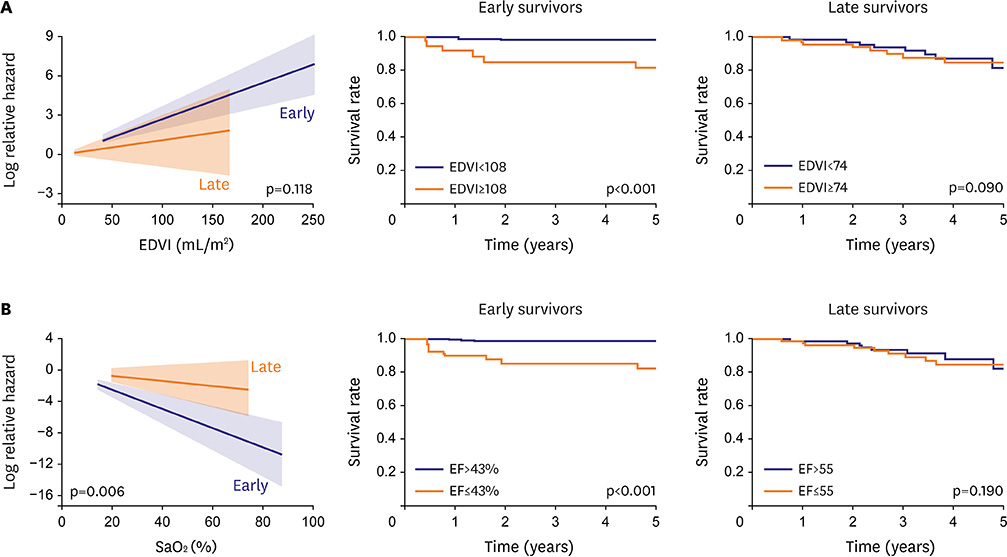
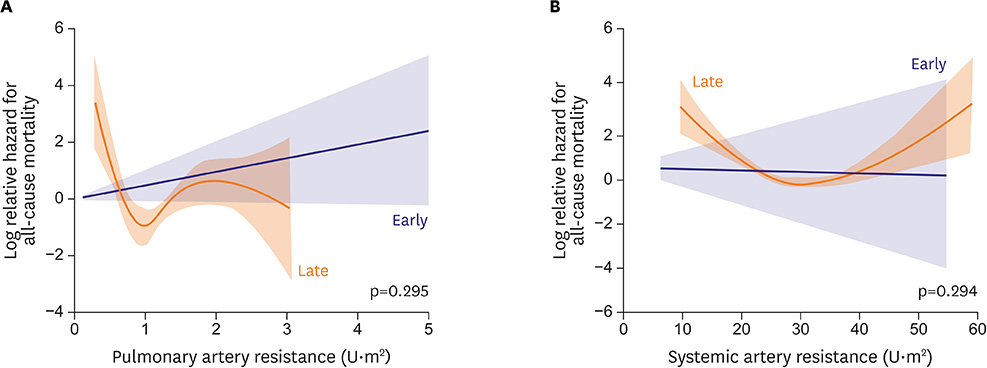
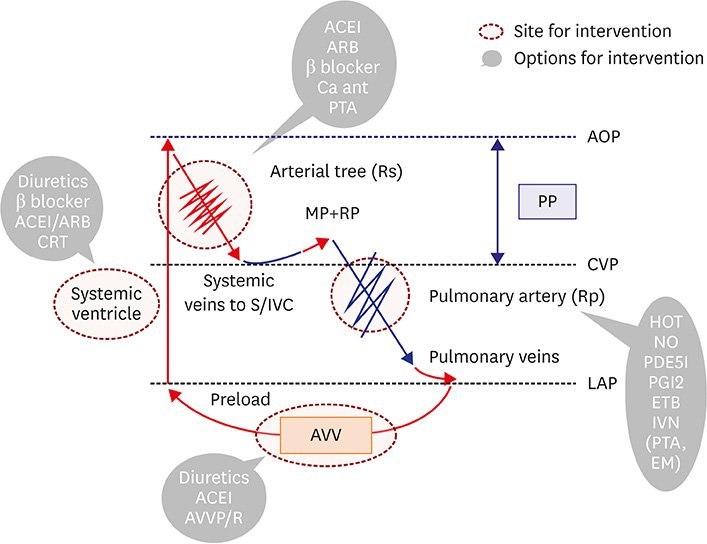
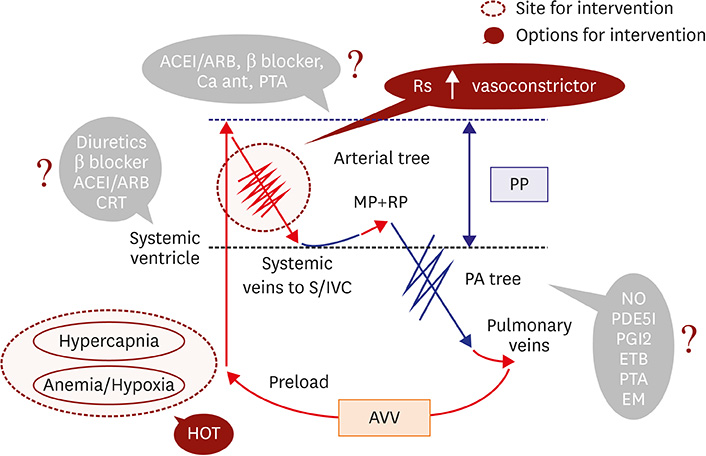
- Fontan circulation is generally characterized by high central venous pressure, low cardiac output, and slightly low arterial oxygen saturation, and it is quite different from normal biventricular physiology. Therefore, when a patient with congenital heart disease is selected as a candidate for this type of circulation, the ultimate goals of therapy consist of 2 components. One is a smooth adjustment to the new circulation, and the other is long-term circulatory stabilization after adjustment. When either of these goals is not achieved, the patient is categorized as having "failed" Fontan circulation, and the prognosis is dismal. For the first goal of smooth adjustment, a lot of effort has been made to establish criteria for patient selection and intensive management immediately after the Fontan operation. For the second goal of long-term circulatory stabilization, there is limited evidence of successful strategies for long-term hemodynamic stabilization. Furthermore, there have been no data on optimal hemodynamics in Fontan circulation that could be used as a reference for patient management. Although small clinical trials and case reports are available, the results cannot be generalized to the majority of Fontan survivors. We recently reported the clinical and hemodynamic characteristics of early and late failing Fontan survivors and their association with all-cause mortality. This knowledge could provide insight into the complex Fontan pathophysiology and might help establish a management strategy for long-term hemodynamic stabilization.
MeSH Terms
Figure
Reference
-
1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971; 26:240–248.2. d’Udekem Y, Iyengar AJ, Cochrane AD, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007; 116:I157–I164.3. Ohuchi H, Kagisaki K, Miyazaki A, et al. Impact of the evolution of the Fontan operation on early and late mortality: a single-center experience of 405 patients over 3 decades. Ann Thorac Surg. 2011; 92:1457–1466.4. Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008; 117:85–92.5. Diller GP, Kempny A, Alonso-Gonzalez R, et al. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation. 2015; 132:2118–2125.6. Quinton E, Nightingale P, Hudsmith L, et al. Prevalence of atrial tachyarrhythmia in adults after Fontan operation. Heart. 2015; 101:1672–1677.7. John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol. 2014; 64:54–62.8. Ohuchi H, Yasuda K, Miyazaki A, et al. Haemodynamic characteristics before and after the onset of protein losing enteropathy in patients after the Fontan operation. Eur J Cardiothorac Surg. 2013; 43:e49–e57.9. Moore JW, Kirby WC, Madden WA, Gaither NS. Development of pulmonary arteriovenous malformations after modified Fontan operations. J Thorac Cardiovasc Surg. 1989; 98:1045–1050.10. Ohuchi H, Yasuda K, Miyazaki A, et al. Prevalence and predictors of haemostatic complications in 412 Fontan patients: their relation to anticoagulation and haemodynamics. Eur J Cardiothorac Surg. 2015; 47:511–519.11. Egbe AC, Connolly HM, Niaz T, et al. Prevalence and outcome of thrombotic and embolic complications in adults after Fontan operation. Am Heart J. 2017; 183:10–17.12. Ohuchi H, Yasuda K, Miyazaki A, et al. Comparison of prognostic variables in children and adults with Fontan circulation. Int J Cardiol. 2014; 173:277–283.13. Assenza GE, Graham DA, Landzberg MJ, et al. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013; 99:491–496.14. Pundi K, Pundi KN, Kamath PS, et al. Liver disease in patients after the Fontan operation. Am J Cardiol. 2016; 117:456–460.15. Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med. 2013; 368:1756–1757.16. Ohuchi H. Adult patients with Fontan circulation: what we know and how to manage adults with Fontan circulation? J Cardiol. 2016; 68:181–189.17. Ohuchi H, Ohashi H, Takasugi H, Yamada O, Yagihara T, Echigo S. Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after Fontan operation. Pediatr Cardiol. 2004; 25:513–521.18. Ohuchi H, Yasuda K, Hasegawa S, et al. Influence of ventricular morphology on aerobic exercise capacity in patients after the Fontan operation. J Am Coll Cardiol. 2001; 37:1967–1974.19. Heinemann M, Breuer J, Steger V, Steil E, Sieverding L, Ziemer G. Incidence and impact of systemic venous collateral development after Glenn and Fontan procedures. Thorac Cardiovasc Surg. 2001; 49:172–178.20. Levine TB, Levine AB. Regional blood flow supply and demand in heart failure. Am Heart J. 1990; 120:1547–1551.21. Drexler H. Reduced exercise tolerance in chronic heart failure and its relationship to neurohumoral factors. Eur Heart J. 1991; 12:Suppl C. 21–28.22. Latus H, Gummel K, Diederichs T, et al. Aortopulmonary collateral flow is related to pulmonary artery size and affects ventricular dimensions in patients after the Fontan procedure. PLoS One. 2013; 8:e81684.23. Ohuchi H, Miyazaki A, Negishi J, et al. Hemodynamic determinants of mortality after Fontan operation. Am Heart J. 2017; 189:9–18.24. Benedict CR, Shelton B, Johnstone DE, et al. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation. 1996; 94:690–697.25. Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart. 2012; 98:1098–1104.26. De Rita F, Crossland D, Griselli M, Hasan A. Management of the failing Fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2015; 18:2–6.27. Mori M, Aguirre AJ, Elder RW, et al. Beyond a broken heart: circulatory dysfunction in the failing Fontan. Pediatr Cardiol. 2014; 35:569–579.28. Mizuno M, Ohuchi H, Matsuyama TA, Miyazaki A, Ishibashi-Ueda H, Yamada O. Diverse multi-organ histopathologic changes in a failed Fontan patient. Pediatr Int. 2016; 58:1061–1065.29. Ohuchi H, Negishi J, Hayama Y, Miyazaki A, Shiraishi I, Ichikawa H. Renal resistive index reflects Fontan pathophysiology and predicts mortality. [Epub ahead of print]. Heart. 2017.30. Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009; 158:30–39.31. Avitabile CM, Leonard MB, Zemel BS, et al. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart. 2014; 100:1702–1707.32. Avitabile CM, Goldberg DJ, Zemel BS, et al. Deficits in bone density and structure in children and young adults following Fontan palliation. Bone. 2015; 77:12–16.33. Book WM, Gerardin J, Saraf A, Marie Valente A, Rodriguez F 3rd. Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis. 2016; 11:296–308.34. Hebson CL, McCabe NM, Elder RW, et al. Hemodynamic phenotype of the failing Fontan in an adult population. Am J Cardiol. 2013; 112:1943–1947.35. Mori M, Hebson C, Shioda K, et al. Catheter-measured hemodynamics of adult Fontan circulation: associations with adverse event and end-organ dysfunctions. Congenit Heart Dis. 2016; 11:589–597.36. Mitchell MB, Campbell DN, Ivy D, et al. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg. 2004; 128:693–702.37. Henaine R, Vergnat M, Bacha EA, et al. Effects of lack of pulsatility on pulmonary endothelial function in the Fontan circulation. J Thorac Cardiovasc Surg. 2013; 146:522–529.38. Ohuchi H, Ono S, Tanabe Y, et al. Long-term serial aerobic exercise capacity and hemodynamic properties in clinically and hemodynamically good, “excellent”, Fontan survivors. Circ J. 2012; 76:195–203.39. Iwakiri Y, Tsai MH, McCabe TJ, et al. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am J Physiol Heart Circ Physiol. 2002; 282:H2084–H2090.40. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006; 43:S121–S131.41. Song D, Liu H, Sharkey KA, Lee SS. Hyperdynamic circulation in portal-hypertensive rats is dependent on central c-fos gene expression. Hepatology. 2002; 35:159–166.42. Waypa GB, Schumacker PT. Hypoxia-induced changes in pulmonary and systemic vascular resistance: where is the O2 sensor? Respir Physiol Neurobiol. 2010; 174:201–211.43. Rychik J, Veldtman G, Rand E, et al. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012; 33:1001–1012.44. Carins TA, Shi WY, Iyengar AJ, et al. Long-term outcomes after first-onset arrhythmia in Fontan physiology. J Thorac Cardiovasc Surg. 2016; 152:1355–1363.e1.45. Pundi KN, Pundi KN, Johnson JN, et al. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis. 2017; 12:17–23.46. Li D, Fan Q, Hirata Y, Ono M, An Q. Arrhythmias after Fontan operation with intra-atrial lateral tunnel versus extra-cardiac conduit: a systematic review and meta-analysis. Pediatr Cardiol. 2017; 38:873–880.47. Allen KY, Downing TE, Glatz AC, et al. Effect of Fontan-associated morbidities on survival with intact Fontan circulation. Am J Cardiol. 2017; 119:1866–1871.48. Nair AP, Timoh T, Fuster V. Contemporary medical management of systolic heart failure. Circ J. 2012; 76:268–277.49. Wojnowich K, Korabathina R. Heart failure update: outpatient management. FP Essent. 2016; 442:18–25.50. Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014; 370:1694–1701.51. Enomoto Y, Aoki M, Nakamura Y, Hagino I, Fujiwara T, Nakajima H. Successful Fontan completion after cardiac resynchronization therapy. Circulation. 2012; 125:e655–e658.52. Takeuchi D, Asagai S, Ishihara K, Nakanishi T. Successful Fontan conversion combined with cardiac resynchronization therapy for a case of failing Fontan circulation with ventricular dysfunction. Eur J Cardiothorac Surg. 2014; 46:913–915.53. Honjo O, Atlin CR, Mertens L, et al. Atrioventricular valve repair in patients with functional single-ventricle physiology: impact of ventricular and valve function and morphology on survival and reintervention. J Thorac Cardiovasc Surg. 2011; 142:326–335.e2.54. King G, Gentles TL, Winlaw DS, et al. Common atrioventricular valve failure during single ventricle palliation. Eur J Cardiothorac Surg. 2017; 51:1037–1043.55. Agnoletti G, Gala S, Ferroni F, et al. Endothelin inhibitors lower pulmonary vascular resistance and improve functional capacity in patients with Fontan circulation. J Thorac Cardiovasc Surg. 2017; 153:1468–1475.56. Tabarsi N, Guan M, Simmonds J, et al. Meta-analysis of the effectiveness of heart transplantation in patients with a failing Fontan. Am J Cardiol. 2017; 119:1269–1274.57. Matsuda H, Ichikawa H, Ueno T, Sawa Y. Heart transplantation for adults with congenital heart disease: current status and future prospects. Gen Thorac Cardiovasc Surg. 2017; 65:309–320.58. de Mattos ÁZ, de Mattos AA, Méndez-Sánchez N. Hepatorenal syndrome: current concepts related to diagnosis and management. Ann Hepatol. 2016; 15:474–481.59. Ruiz-del-햞bol L. Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015; 21:11502–11521.60. Uzun O, Wong JK, Bhole V, Stumper O. Resolution of protein-losing enteropathy and normalization of mesenteric Doppler flow with sildenafil after Fontan. Ann Thorac Surg. 2006; 82:e39–e40.61. Bhagirath KM, Tam JW. Resolution of protein-losing enteropathy with low-molecular weight heparin in an adult patient with Fontan palliation. Ann Thorac Surg. 2007; 84:2110–2112.62. Hoashi T, Ichikawa H, Ueno T, Kogaki S, Sawa Y. Steroid pulse therapy for protein-losing enteropathy after the Fontan operation. Congenit Heart Dis. 2009; 4:284–287.63. Straver B, Wagenaar LJ, Blom NA, et al. Percutaneous tricuspid valve implantation in a Fontan patient with congestive heart failure and protein-losing enteropathy. Circ Cardiovasc Interv. 2011; 4:112–113.64. John AS, Driscoll DJ, Warnes CA, Phillips SD, Cetta F. The use of oral budesonide in adolescents and adults with protein-losing enteropathy after the Fontan operation. Ann Thorac Surg. 2011; 92:1451–1456.65. Okano S, Sugimoto M, Takase M, Iseki K, Kajihama A, Azuma H. Effectiveness of high-dose spironolactone therapy in a patient with recurrent protein-losing enteropathy after the Fontan procedure. Intern Med. 2016; 55:1611–1614.66. António M, Gordo A, Pereira C, Pinto F, Fragata I, Fragata J. Thoracic duct decompression for protein-losing enteropathy in failing Fontan circulation. Ann Thorac Surg. 2016; 101:2370–2373.67. Friedland-Little JM, Gajarski RJ, Schumacher KR. Dopamine as a potential rescue therapy for refractory protein-losing enteropathy in Fontan-palliated patients. Pediatr Transplant. 2017; 21:e12925.68. Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med. 2005; 6:76–78.69. Apostolopoulou SC, Papagiannis J, Rammos S. Bosentan induces clinical, exercise and hemodynamic improvement in a pre-transplant patient with plastic bronchitis after Fontan operation. J Heart Lung Transplant. 2005; 24:1174–1176.70. Heath L, Ling S, Racz J, et al. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011; 32:1182–1189.71. Dori Y, Keller MS, Rome JJ, et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation. 2016; 133:1160–1170.72. Opocher F, Varnier M, Sanders SP, et al. Effects of aerobic exercise training in children after the Fontan operation. Am J Cardiol. 2005; 95:150–152.73. Brassard P, Poirier P, Martin J, et al. Impact of exercise training on muscle function and ergoreflex in Fontan patients: a pilot study. Int J Cardiol. 2006; 107:85–94.74. Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008; 29:1681–1687.75. Goldberg DJ, French B, McBride MG, et al. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011; 123:1185–1193.76. Schuuring MJ, Vis JC, van Dijk AP, et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. Eur J Heart Fail. 2013; 15:690–698.77. Cordina RL, O'Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013; 168:780–788.78. Rhodes J, Ubeda-Tikkanen A, Clair M, et al. Effect of inhaled iloprost on the exercise function of Fontan patients: a demonstration of concept. Int J Cardiol. 2013; 168:2435–2440.79. Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging. 2014; 7:265–273.80. Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (treatment with endothelin receptor antagonist in Fontan patients, a randomized, placebo-controlled, double-blind study measuring peak oxygen consumption) study. Circulation. 2014; 130:2021–2030.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Protein-losing Enteropathy After Fontan Procedure by Conversion to the Total Cavopulmonary Connection with Fenestration
- CT and MRI Evaluation of the Fontan Pathway: Pearls and Pitfalls
- Mid- and Long Term Outcome of Fontan Procedure: Extracardiac Conduit Fontan versus Lateral Tunnel Fontan
- Computed Tomography in the Evaluation of Fontan Circulation
- Anesthetic management of laparoscopic pheochromocytoma excision in a patient with a Fontan circulation: a case report