Prognostic factors after curative resection hepatocellular carcinoma and the surgeon's role
- Affiliations
-
- 1Department of Surgery, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- 2Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. kimdg@catholic.ac.kr
- 3Department of Medical Life Science, The Catholic University of Korea, Seoul, Korea.
- KMID: 2393990
- DOI: http://doi.org/10.4174/astr.2017.93.5.252
Abstract
- PURPOSE
Patient, surgical, and tumor factors affect the outcome after surgical resection for hepatocellular carcinoma (HCC). The surgical factors are only modifiable by the surgeon. We reviewed our experience with curative resection for HCC in terms of surgical factors.
METHODS
After analyses of the prospectively collected clinical data of 256 consecutive patients undergoing surgical resection for HCC, prognostic factors for disease-free survival (DFS) and overall survival (OS) were identified; all patients were stratified by tumor diameters > or <5 cm and their outcomes were compared.
RESULTS
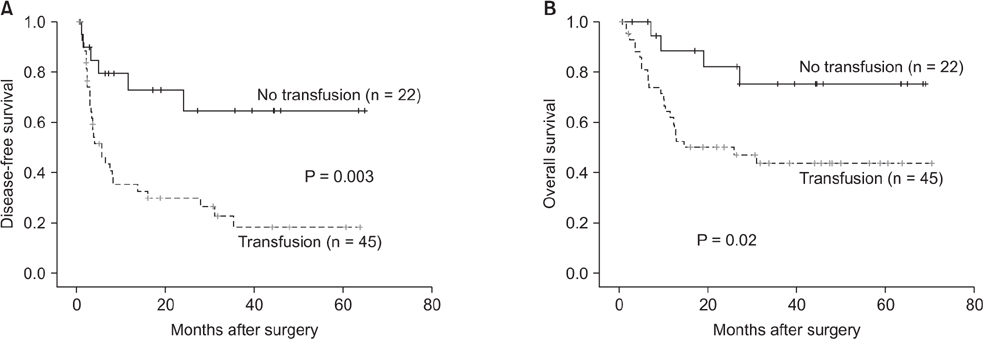
Multivariate analyses showed that microvascular invasion, estimated blood loss, blood transfusion, and the number of tumors were independent adverse prognostic factors for DFS, whereas microvascular invasion, serum alpha fetoprotein, and tumor diameter were independent adverse prognostic factors for OS. Blood transfusion had borderline significance (P = 0.076). After stratification by tumor diameter, blood transfusion was only associated with poor DFS and OS in patients with tumor diameters > 5 cm.
CONCLUSION
Tumor recurrence after liver resection for HCC depends on tumor status, bleeding, and transfusions, which subsequently lead to poor patient survival. Surgeons can help improve the prognosis of patients by minimizing blood loss and transfusion, particularly in patients with larger tumors.
MeSH Terms
Figure
Cited by 2 articles
-
Staged partial hepatectomy versus transarterial chemoembolization for the treatment of spontaneous hepatocellular carcinoma rupture: a multicenter analysis in Korea
Hyung Soon Lee, Gi Hong Choi, Jin Sub Choi, Kwang-Hyub Han, Sang Hoon Ahn, Do Young Kim, Jun Yong Park, Seung Up Kim, Sung Hoon Kim, Dong Sup Yoon, Jae Keun Kim, Jong Won Choi, Soon Sun Kim, Hana Park
Ann Surg Treat Res. 2019;96(6):275-282. doi: 10.4174/astr.2019.96.6.275.Preoperative lymphocyte-to-C-reactive protein ratio predicts hepatocellular carcinoma recurrence after surgery
Masashi Utsumi, Masaru Inagaki, Koji Kitada, Naoyuki Tokunaga, Midori Kondo, Yuya Sakurai, Kosuke Yunoki, Ryosuke Hamano, Hideaki Miyasou, Yousuke Tsunemitsu, Shinya Otsuka
Ann Surg Treat Res. 2022;103(2):72-80. doi: 10.4174/astr.2022.103.2.72.
Reference
-
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–1127.2. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943.3. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2015; 16:465–522.4. Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011; 253:745–758.5. Han DH, Choi GH, Park JY, Ahn SH, Kim KS, Choi JS, et al. Lesson from 610 liver resections of hepatocellular carcinoma in a single center over 10 years. World J Surg Oncol. 2014; 12:192.6. Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002; 8:193–199.7. Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999; 134:1124–1130.8. Sim HG, Ooi LL. Results of resections for hepatocellular carcinoma in a new hepatobiliary unit. ANZ J Surg. 2003; 73:8–13.9. Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005; 242:252–259.10. Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015; 19:1281–1290.11. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38:200–207.12. Park JH, Koh KC, Choi MS, Lee JH, Yoo BC, Paik SW, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg. 2006; 192:29–33.13. Tanaka K, Shimada H, Matsumoto C, Matsuo K, Nagano Y, Endo I, et al. Anatomic versus limited nonanatomic resection for solitary hepatocellular carcinoma. Surgery. 2008; 143:607–615.14. Zhang XF, Meng B, Qi X, Yu L, Liu C, Liu XM, et al. Prognostic factors after liver resection for hepatocellular carcinoma with hepatitis B virus-related cirrhosis: surgeon’s role in survival. Eur J Surg Oncol. 2009; 35:622–628.15. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer. 1994; 74:2772–2780.16. Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004; 101:796–802.17. Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998; 82:1028–1036.18. Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000; 231:544–551.19. Adachi E, Maeda T, Matsumata T, Shirabe K, Kinukawa N, Sugimachi K, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995; 108:768–775.20. Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991; 214:114–117.21. Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med. 1994; 118:371–379.22. Gascon P, Zoumbos NC, Young NS. Immunologic abnormalities in patients receiving multiple blood transfusions. Ann Intern Med. 1984; 100:173–177.23. Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984; 64:308–310.24. Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999; 23:676–680.25. Hanazaki K, Kajikawa S, Shimozawa N, Matsushita A, Machida T, Shimada K, et al. Perioperative blood transfusion and survival following curative hepatic resection for hepatocellular carcinoma. Hepatogastroenterology. 2005; 52:524–529.26. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000; 232:10–24.27. Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003; 10:189–194.28. Man K, Ng KT, Lo CM, Ho JW, Sun BS, Sun CK, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases: activation of cell invasion and migration pathways. Liver Transpl. 2007; 13:1669–1677.29. Ozaki M, Todo S. Surgical stress and tumor behavior: impact of ischemia-reperfusion and hepatic resection on tumor progression. Liver Transpl. 2007; 13:1623–1626.30. Shimoda M, Iwasaki Y, Sawada T, Kubota K. Protective effect of ischemic preconditioning against liver injury after major hepatectomy using the intermittent pringle maneuver in swine. Pathobiology. 2007; 74:42–49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of prognostic nutritional index on the recurrence of hepatocellular carcinoma after a curative resection
- Prognostic Factors and Clinicopathologic Features after Resection of Small Hepatocellular Carcinoma (< or =2 cm)
- Prognosis after intrahepatic recurrence in the patients who underwent curative resection for hepatocellular carcinoma
- The Predictive Factors of Recurrence in Resected Hepatocellular Carcinoma
- Surgical outcome and prognostic factors in patients with gallbladder carcinoma