Nat Prod Sci.
2017 Sep;23(3):192-200. 10.20307/nps.2017.23.3.192.
Antioxidant and Antiaging Assays of Hibiscus sabdariffa Extract and Its Compounds
- Affiliations
-
- 1Medical Research Center, Faculty of Medicine, Maranatha Christian University, Jl. Prof. Drg. Surya Sumantri No. 65 Bandung 40164, West Java, Indonesia. wahyu_w60@yahoo.com
- 2Aretha Medika Utama, Biomolecular and Biomedical Research Center, Jl. Babakan Jeruk 2, No. 9, Bandung 40163, West Java, Indonesia.
- KMID: 2393800
- DOI: http://doi.org/10.20307/nps.2017.23.3.192
Abstract
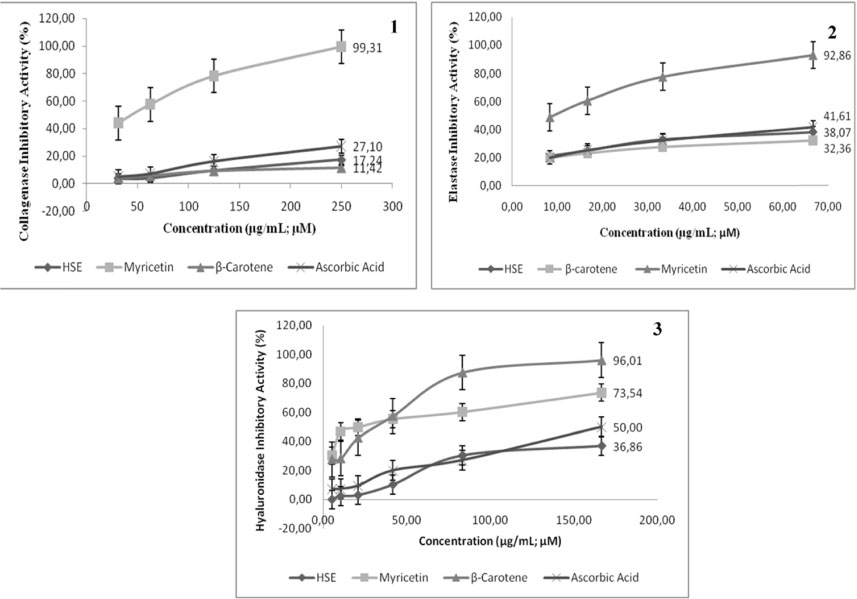
- Skin aging is a complex biological process due to intrinsic and extrinsic factors. Free radical oxidative is one of extrinsic factors that induce activation of collagenase, elastase and hyaluronidase. Natural product from plants has been used as antioxidant and antiaging. This study aimed to evaluate antioxidant and antiaging properties of Hibiscus sabdariffa extract (HSE) and its compounds including myricetin, ascorbic acid, and β carotene. The phytochemical of H. sabdariffa was determined using modified Farnsworth method and presence of phenols, flavonoids and tannins were in moderate content, whereas triterpenoids and alkaloids were in low content. Total phenolic content performed using Folin-Ciocalteu method, was 23.85 µg GAE/mg. Quantitative analysis of myricetin, β-carotene, and ascorbic acid of HSE was performed with Ultra-High Performance Liquid Chromatography (UHPLC) that shows 78.23 µg/mg myricetin, 0.034 µg/mg β-carotene, whilst ascorbic acid was not detected. HSE has lower activity on DPPH (ICâ‚…â‚€ = 195.73 µg/mL) compared to β-carotene, the lowest in ABTS assay (IC50 = 74.58 µg/mL) and low activity in FRAP assay (46.24 µM Fe(II)/µg) compared to myricetin, β-carotene. Antiaging was measured through inhibitory activity of collagenase, elastase, and hyaluronidase. HSE had weakest collagenase inhibitory activity (ICâ‚…â‚€= 750.33 µg/mL), elastase inhibitory activity (103.83 µg/mL), hyaluronidase inhibitory activity (ICâ‚…â‚€ = 619.43 µg/mL) compared to myricetin, β-carotene, and ascorbic acid. HSE contain higher myricetin compared to β-carotene. HSE has moderate antioxidants and lowest antiaging activities. Myricetin is the most active both antioxidant and antiaging activities.
Keyword
MeSH Terms
-
Alkaloids
Antioxidants
Ascorbic Acid
Biological Processes
Carotenoids
Chromatography, Liquid
Collagenases
Flavonoids
Hibiscus*
Hyaluronoglucosaminidase
Methods
Pancreatic Elastase
Phenol
Phenols
Skin Aging
Tannins
Alkaloids
Antioxidants
Ascorbic Acid
Carotenoids
Collagenases
Flavonoids
Hyaluronoglucosaminidase
Pancreatic Elastase
Phenol
Phenols
Tannins
Figure
Reference
-
1. Benaiges A, Marcet P, Armengol R, Betes C, Gironés E. Int J Cosmet Sci. 1998; 20:223–233.2. Jenkins G. Mech Ageing Dev. 2002; 123:801–810.3. Schlotmann K, Kaeten M, Black AF, Damour O, Waldmann-Laue M, Förster T. Int J Cosmet Sci. 2001; 23:309–318.4. McCullough JL, Kelly KM. Ann N Y Acad Sci. 2006; 1067:323–331.5. Aslam MN, Lansky EP, Varani J. J Ethnopharmacol. 2006; 103:311–318.6. Chanchal D, Swarnlata S. J Cosmet Dermatol. 2008; 7:89–95.7. Farris PK. Cosmetic Dermatol. 2003; 16:59–72.8. Roy A, Sahu RK, Matlam M, Deshmukh VK, Dwivedi J, Jha AK. Pharmacogn Rev. 2013; 7:97–106.9. Pandel R, Poljšak B, Godic A, Dahmane R. ISRN Dermatol. 2013; 2013:930164.10. Taniguchi M, Arai N, Kohno K, Ushio S, Fukuda S. Eur J Pharmacol. 2012; 674:126–131.11. Johnston MF, Hili P, Naughton DP. BMC Complement Altern Med. 2009; 9:1–11.12. Tsai PJ, McIntosh J, Pearce P, Camden B, Jordan B. Food Res Int. 2002; 35:351–356.13. Mohamad O, Mohd B, Abdul M, Herman S. Bull PGM. 2002.14. Widowati W, Wijaya L, Wargasetia TL, Bachtiar I, Yellianty Y, Laksmitawati DR. J Exp Integr Med. 2013; 3:225–230.15. Widowati W, Ratnawati H, Rusdi U, Winarno W, Imanuel V. HAYATI J Biosci. 2010; 17:85–90.
Article16. Bera TK, Chatterjee K, Ghosh D. Int J Ayurveda Res. 2010; 1:18–24.17. Adnyana IK, Abuzaid AS, Iskandar EY, Kurniati NF. . Int J Med Res Health Sci. 2016; 5:23–28.18. Widowati W, Ratnawati H, Husin W, Maesaroh M. Biomed Eng. 2015; 1:24–29.19. Widowati W, Herlina T, Ratnawati H, Constantia G, Deva I, Maesaroh M. Biol Med Nat Prod Chem. 2015; 4:35–39.20. Lucini L, Pellizzoni M, Baffi C, Molinari GP. J Sci Food Agric. 2012; 92:1297–1303.21. Sohn DH, Kim YC, Oh SH, Park EJ, Li X, Lee BH. Phytomedicine. 2003; 10:165–169.22. Dasgupta A, Ray D, Chatterjee A, Roy A, Acharya K. J Pharm Biol Chem Sci. 2014; 5:510–520.23. Widowati W, Widyanto RM, Husin W, Ratnawati H, Laksmitawati DR, Setiawan B, Nugrahenny D, Bachtiar I. Iran J Basic Med Sci. 2014; 17:702–709.24. Mishra A, Bapat MM, Tilak JC, Devasagayam TT. Curr Sci. 2006; 91:90–93.25. Widowati W, Fauziah N, Herdiman H, Afni M, Afifah E, Kusuma HSW, Nufus H, Arumwardana S, Rihibiha DD. J Nat Rem. 2016; 16:89–99.26. Tu PT, Tawata S. Molecules. 2015; 20:16723–16740.27. Bergmeier D, Berres PHD, Filippi D, Bilibio D, Bettiol VR, Priamo WL. Maringa. 2014; 36:545–551.28. Mahadevan N, Shivali , Kamboj P. Nat Prod Radiance. 2009; 8:77–83.29. Fusco D, Colloca G, Lo Monaco MR, Cesari M. Clin Interv Aging. 2007; 2:377–387.30. Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Food Chem. 2004; 84:551–562.31. Oliveira CP, Kassab P, Lopasso FP, Souza HP, Janiszewski M, Laurindo FR, Iriya K, Laudanna AA. World J Gastroenterol. 2003; 9:446–448.32. Wang X, Falcone T, Attaran M, Goldberg JM, Agarwal A, Sharma RK. Fertil Steril. 2002; 78:1272–1277.33. Huang JH, Huang CC, Fang JY, Yang C, Chan CM, Wu NL, Kang SW, Hung CF. Toxicol In Vitro. 2010; 24:21–28.34. Formica JV, Regelson W. Food Chem Toxicol. 1995; 33:1061–1080.35. Sim GS, Lee BC, Cho HS, Lee JW, Kim JH, Lee DH, Kim JH, Pyo HB, Moon DC, Oh KW, Yun YP, Hong JT. Arch Pharm Res. 2007; 30:290–298.36. Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. J Agric Food Chem. 2006; 54:9798–9804.37. Mattivi F, Guzzon R, Vrhovsek U, Stefanini M, Velasco R. J Agric Food Chem. 2006; 54:7692–7702.38. Swindells K, Rhodes L. Photodermatol Photoimmunol Photomed. 2004; 20:297–304.39. Boelsma E, Hendriks H, Roza L. Am J Clin Nutr. 2001; 73:853–864.40. Haftek M, Mac-Mary S, Le Bitoux MA, Creidi P, Seité S, Rougier A, Humbert P. Exp Dermatol. 2008; 17:946–952.41. Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. Biochim Biophys Acta. 2000; 1478:51–60.42. Pientaweeratch S, Panapisal V, Tansirikongkol A. Pharm Biol. 2016; 54:1865–1872.43. Roy A, Sahu RK, Matlam M, Deshmukh VK, Dwivedi J, Jha AK. Pharmacogn Rev. 2013; 7:97–106.44. Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, Heo YS, Kang NJ, Bode AM, Dong Z, Lee HJ. Biochem Pharmacol. 2010; 79:1455–1461.45. Kanashiro A, Souza JG, Kabeya LM, Azzolini AE, Lucisano-Valim YM. Z Naturforsch C. 2007; 62:357–361.46. Sahasrabudhe A, Deodhar M. Int J Bot. 2010; 6:299–303.47. Getoff N. Rad Phys Chem. 2007; 76:1577–1586.48. Kammerer C, Czermak I, Getoff N. Radiat Phys Chem. 2001; 60:71–72.49. Darr D, Dunston S, Faust H, Pinnell S. Acta Derm Venereol. 1996; 76:264–268.50. Lin JY, Selim MA, Shea CR, Grichnik JM, Omar MM, Monteiro-Riviere NA, Pinnell SR. J Am Acad Dermatol. 2003; 48:866–874.51. Rizvi S, Jha R. Expert Opin Drug Discov. 2011; 6:89–102.52. Girish KS, Kemparaju K. Life Sci. 2007; 80:1921–1943.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Occurrence of Root Rot and Vascular Wilt Diseases in Roselle (Hibiscus sabdariffa L.) in Upper Egypt
- Novel Insight into the Cellular and Molecular Signalling Pathways on Cancer Preventing Effects of Hibiscus sabdariffa: A Review
- Nonanoic Acid, an Antifungal Compound from Hibiscus syriacus Ggoma
- Antioxidant Compounds Isolated from the Roots of Phlomis umbrosa Turcz.
- The antioxidant and cytotoxic activities of Sonchus oleraceus L. extracts