Nat Prod Sci.
2017 Sep;23(3):169-174. 10.20307/nps.2017.23.3.169.
Quercetin Relaxed the Smooth Muscle of Rabbit Penile Corpus Cavernosum by Activating the NO-cGMP Signaling Pathway
- Affiliations
-
- 1Department of Urology, Chonbuk National University and Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute and Clinical Trial Center of Medical Device of Chonbuk National University, 20, Geonji-ro, Deokjin-gu, Jeonju 54896, Korea. rain@chonbuk.ac.kr
- 2College of Pharmacy, Kyungsung University, 309 Suyeong-ro, Nam-gu, Busan 48434, Korea. fiona30@ks.ac.kr
- KMID: 2393797
- DOI: http://doi.org/10.20307/nps.2017.23.3.169
Abstract
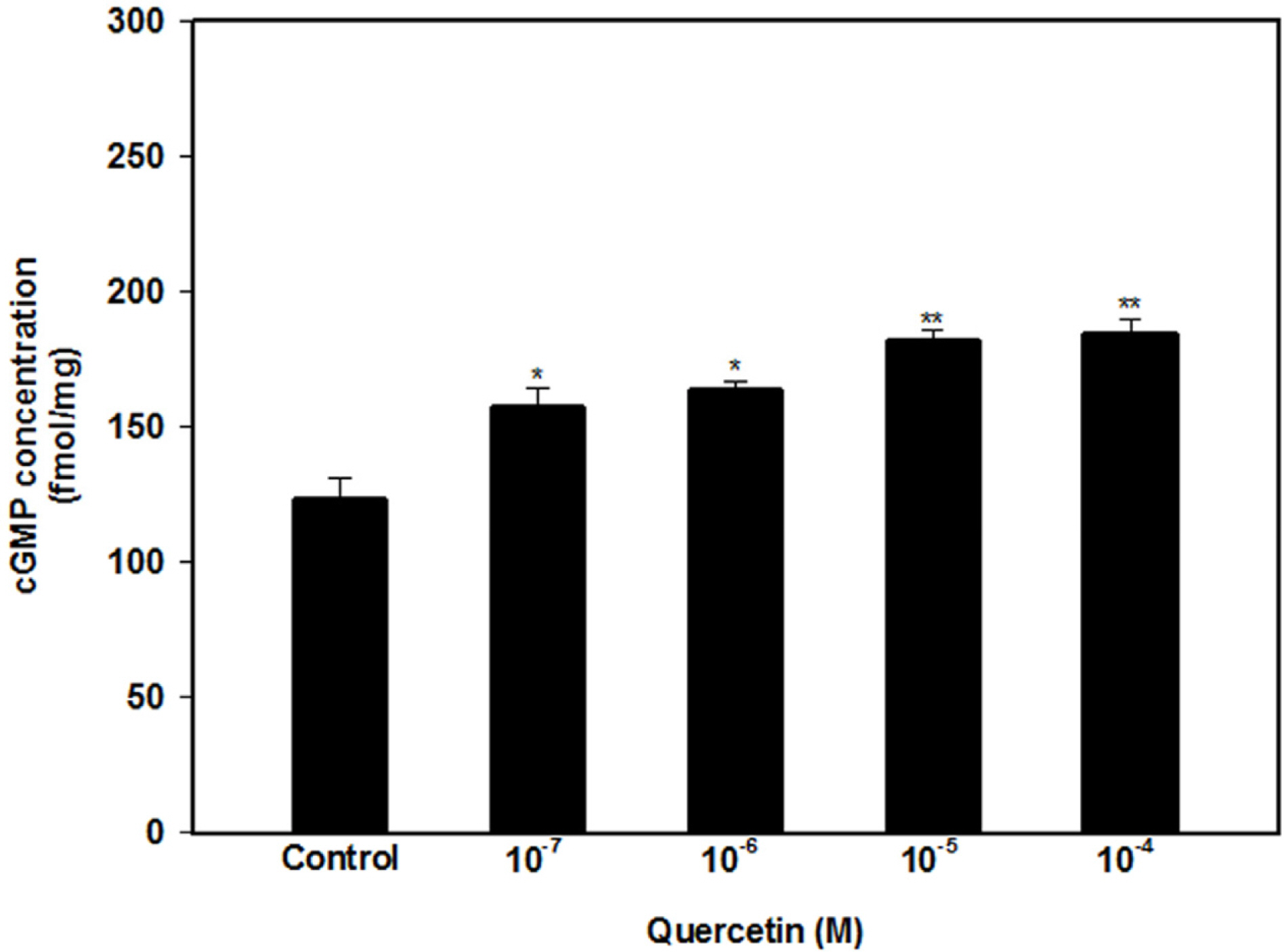
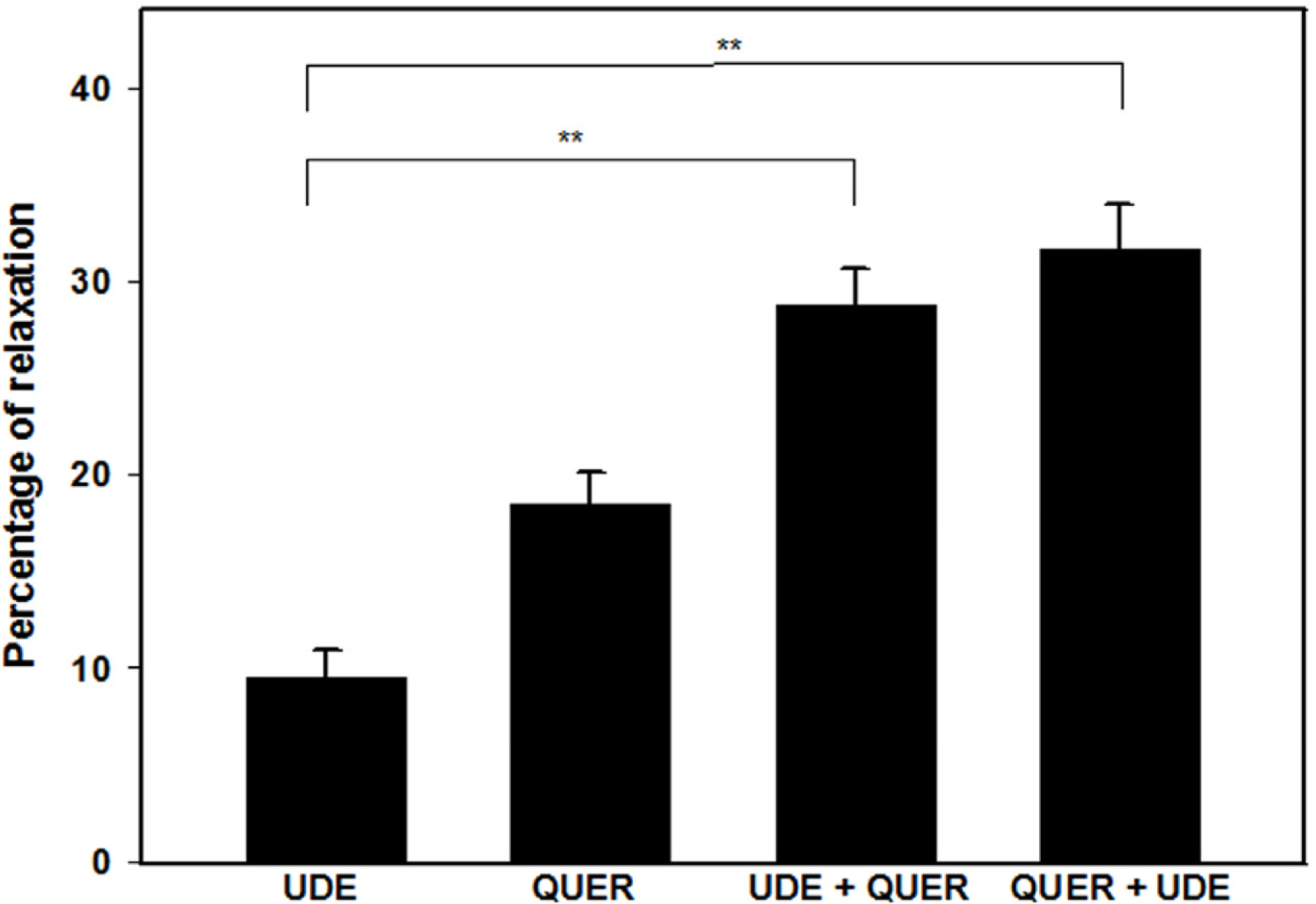
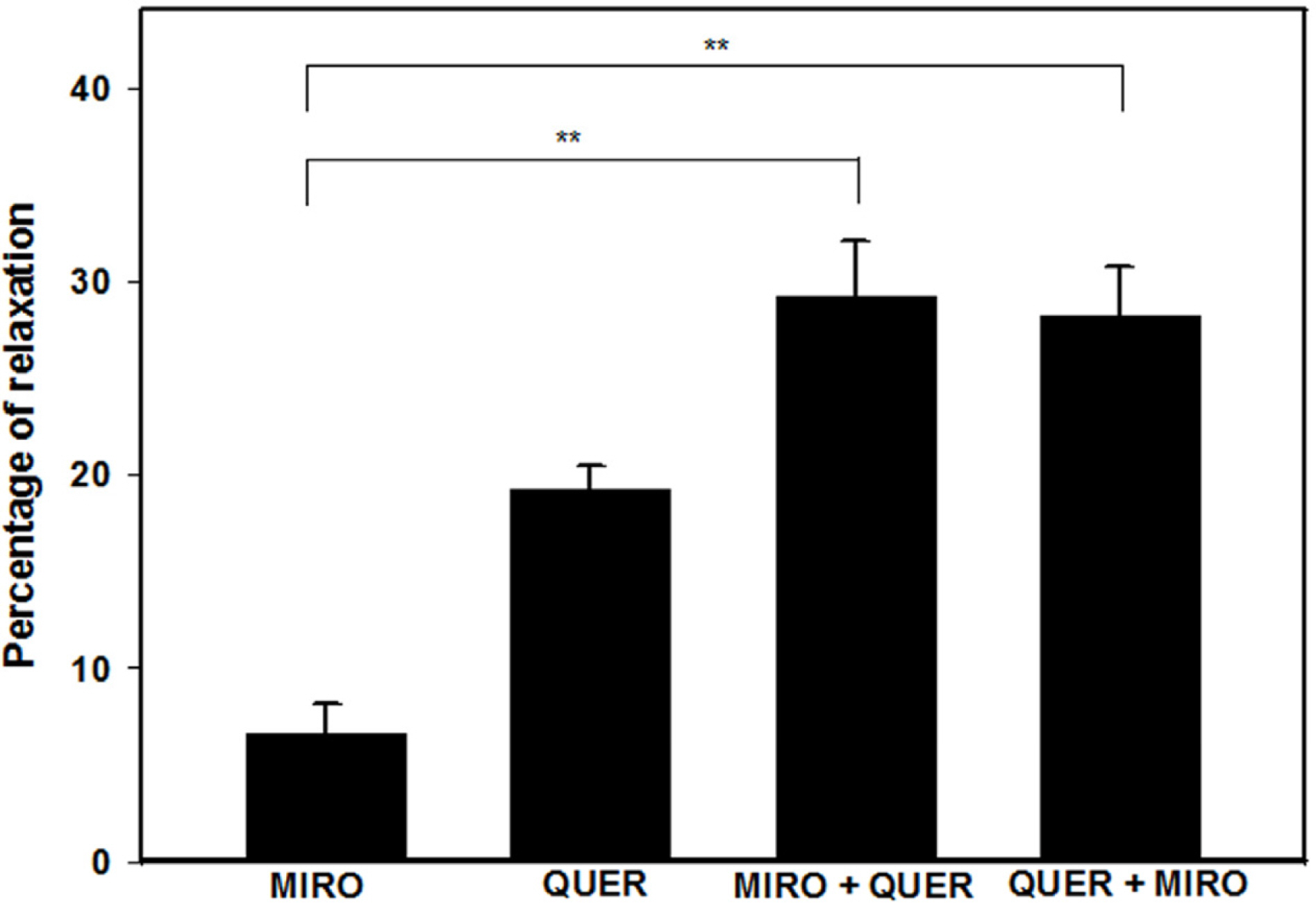
- The aim of this study was to investigate the effect and action mechanism of quercetin on penile corpus cavernosum smooth muscle (PCCSM). PCCSM precontracted with phenylephrine (Phe) was treated with four different concentrations of quercetin (10−7, 10−6, 10−5 and 10−4 M). PCCSM were preincubated with N-Nitro-L-arginine methyl ester hydrochloride (L-NAME) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) to block nitric oxide synthase and guanylate cyclase, respectively. The changes in PCCSM tension were recorded, and cyclic nucleotides in the perfusate were measured by radioimmunoassay. The interactions of quercetin with phosphodiesterase type 5 inhibitors (PDE5-Is) such as sildenafil, udenafil and mirodenafil, were also evaluated. PCCSM relaxation induced by quercetin occurred in a concentrationdependent manner. The application of quercetin to PCCSM pre-treated with L-NAME and ODQ significantly inhibited the relaxation. Quercetin significantly increased cGMP in the perfusate. Furthermore, quercetin enhanced PDE5-Is-induced relaxation of PCCSM. Quercetin relaxed the PCCSM by activating the NO-cGMP signaling pathway, and it may be a therapeutic candidate or an alternative treatment for patients with erectile dysfunction who do not completely respond to PDE5-Is.
Keyword
MeSH Terms
-
Erectile Dysfunction
Guanylate Cyclase
Humans
Male
Muscle, Smooth*
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase
Nucleotides, Cyclic
Phenylephrine
Phosphodiesterase 5 Inhibitors
Quercetin*
Radioimmunoassay
Relaxation
Sildenafil Citrate
Guanylate Cyclase
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase
Nucleotides, Cyclic
Phenylephrine
Phosphodiesterase 5 Inhibitors
Quercetin
Sildenafil Citrate
Figure
Reference
-
References
(1). Kouidrat Y.., Zaitouni A.., Amad A.., Diouf M.., Desailloud R.., Loas G.., Lalau J. D. J.Diabetes Complicat. 2017. 31:108–113.(2). Sheweita S. A.., Wally M.., Hassan M.Oxid. Med. Cell Longev. 2016. 2016:1–9.(3). Matos G.., Hirotsu C.., Alvarenga T. A.., Cintra F.., Bittencourt L.., Tufik S.., Andersen M. L.Andrology. 2013. 1:872–878.(4). Hatzichristou D.., Rosen R. C.., Broderick G.., Clayton A.., Cuzin B.., Derogatis L.., Litwin M.., Meuleman E.., O'Leary M.., Quirk F.., Sadovsky R.., Seftel A. J.Sex. Med. 2004. 1:49–57.(5). Chiou W. F.., Chen C. F.Eur. J. Pharmacol. 2002. 446:151–159.(6). Sullivan M. E.., Thompson C. S.., Dashwood M. R.., Khan M. A.., Jeremy J. Y.., Morgan R. J.., Mikhailidis D. P.Cardiovasc. Res. 1999. 43:658–665.(7). Deng W.., Bivalacqua T. J.., Hellstrom W. J. G.., Kadowitz P. J.Int. J. Impot. Res. 2005. 17:S57–S63.(8). Yan L.., Zhang J. D.., Wang B.., Lv Y. J.., Jiang H.., Liu G. L.., Qiao Y.., Ren M.., Guo X. F.PLoS One. 2013. 8:1–14.(9). Bhutada P.., Mundhada Y.., Bansod K.., Bhutada C.., Tawari S.., Dixit P.., Mundhada D.Neurobiol. Learn. Mem. 2010. 94:293–302.(10). Ranawat P.., Pathak C. M.., Khanduja K. L.Phytother. Res. 2013. 27:802–810.(11). Senggunprai L.., Kukongviriyapan V.., Prawan A.., Kukongviriyapan U.Phytother. Res. 2014. 28:841–848.(12). Erden Inal M.., Kahraman A.Toxicology. 2000. 154:21–29.(13). Chen C. K.., Pace-Asciak C. R.Gen. Pharmacol. 1996. 27:363–366.(14). Zhao C.., Chae H. J.., Kim S. H.., Cui W. S.., Lee S. W.., Jeon J. H.., Park J. K. J.Sex. Med. 2010. 7:1419–1428.(15). Cui X.., Lee S. J.., Kim S. Z.., Kim S. H.., Cho K. W.Eur. J. Pharmacol. 2000. 402:129–137.(16). Li X.., Oh H. C.., Son S. B.., Lee Y. J.., Kang D. G.., Lee H. S.Evid. Based Complement. Alternat. Med. 2012. 2012:1–10.(17). Feng X. T.., Qin C. B.., Leng J.., Tang Q. L.., Shi H.., Zhai L. N.., Li S. L.Biosci. Biotechnol. Biochem. 2012. 76:257–263.(18). Nimmegeers S.., Sips P.., Buys E.., Decaluwé K.., Brouckaert P.., Van de Voorde J.Int. J. Impot. Res. 2008. 20:278–284.(19). Eardley I.Br. J. Diabetes Vasc. Dis. 2002. 2:272–276.(20). Hosogai N.., Takakura S.., Manda T.., Mutoh S.Eur. J. Pharmacol. 2003. 473:65–70.(21). Choi B. R.., Kumar S. K.., Zhao C.., Zhang L. T.., Kim C. Y.., Lee S. W.., Jeon J. H.., Soní K. K.., Kim S. H.., Park N. C.., Kim H. K.., Park J. K.Int. J. Impot. Res. 2015. 27:225–232.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect and Mechanism of the Specific Phosphodiesterase (PDE) III-Inhibitor Milrinone on Human and Rabbit Corpus Cavernosum
- Effect of Korean Red Ginseng on the Relaxation of Clitoral Corpus Cavernosum in Rabbit
- Effects of Angiotensin III in Rabbit Corpus Cavernosum Smooth Muscle Contraction: Comparing with Angiotensin I and Angiotensin II
- Mechanism of Action of Endothelium-Dependent Relaxation Substances on Rabbit Corpus Cavernosum
- A study of alpha adrenergic receptors in human and rabbit corpus cavernosum tissue