Investig Magn Reson Imaging.
2017 Sep;21(3):162-170. 10.13104/imri.2017.21.3.162.
The Imaging Features of Desmoid Tumors: the Usefulness of Diffusion Weighted Imaging to Differentiate between Desmoid and Malignant Soft Tissue Tumors
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. hiohsn@gmail.com
- KMID: 2392687
- DOI: http://doi.org/10.13104/imri.2017.21.3.162
Abstract
- PURPOSE
To evaluate the imaging findings of desmoid tumors using various imaging modalities and to evaluate whether diffusion-weighted imaging (DWI) can help differentiate between desmoid and malignant tumors.
MATERIALS AND METHODS
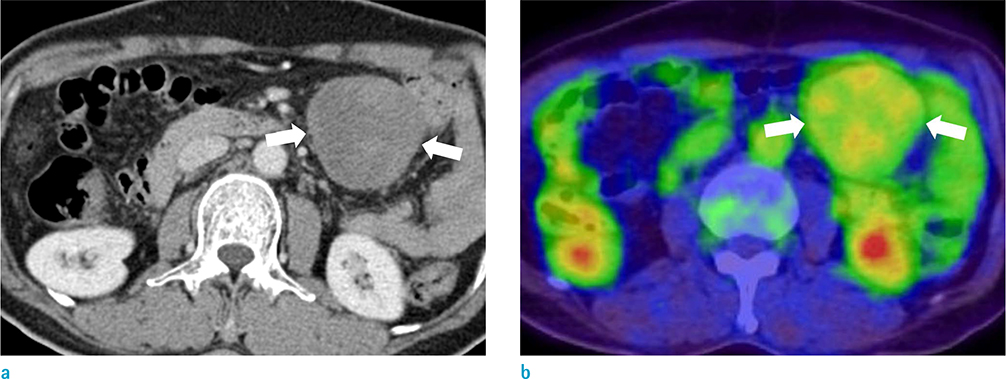
The study included 27 patients with pathologically confirmed desmoid tumors. Two radiologists reviewed 23 computed tomography (CT), 12 magnetic resonance imaging (MRI) and 8 positron emission tomography-computed tomography (PET-CT) scans of desmoid tumors and recorded data regarding the shape, multiplicity, size, location, degree of enhancement, and presence or absence of calcification or hemorrhage. The signal intensity of masses on T1- and T2-weighted imaging and the presence or absence of whirling or band-like low signal intensity on T2-weighted imaging were recorded. The apparent diffusion coefficient (ADC) values of the desmoid tumors in nine patients with DWIs were compared with the ADC values of 32 malignant tumors. The maximum standardized uptake value (SUV(max)) on PET-CT images was measured in 8 patients who underwent a PET-CT.
RESULTS
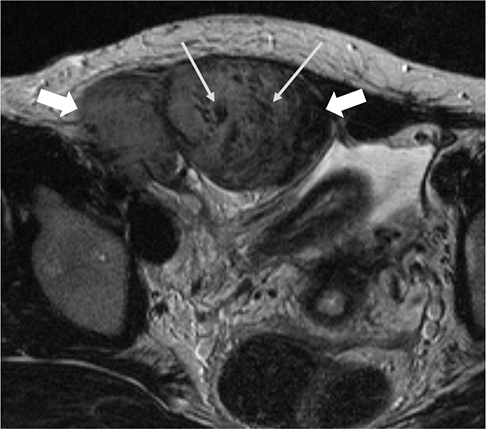
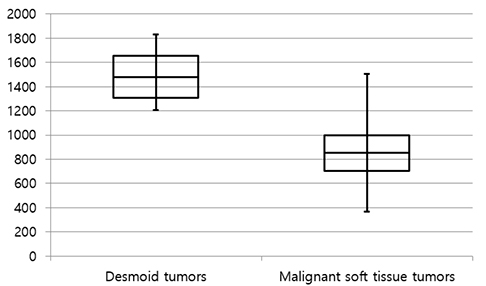
The mean size of the 27 tumors was 6.77 cm (range, 2.5-26 cm) and four tumors exhibited multiplicity. The desmoid tumors were classified by shape as either mass forming (n = 18), infiltrative (n = 4), or combined (n = 5). The location of the tumors was either intra-abdominal (n = 15), within the abdominal wall (n = 8) or extra-abdominal (n = 4). Among the 27 tumors, 21 showed moderate to marked enhancement and 22 showed homogeneous enhancement. Two tumors showed calcifications and one displayed hemorrhage. Eleven of the 12 MR T2-weighted images showed whirling or band-like low signal intensity areas in the mass. The mean ADC value of the desmoid tumors (1493 × 10â»â¶ mm²/s) was significantly higher than the mean of the malignant soft tissue tumors (873 × 10â»â¶ mm²/s, P < 0.001). On the PET-CT images, all tumors exhibited an intermediate SUV(max) (mean, 3.7; range, 2.3-4.5).
CONCLUSION
Desmoids tumors showed homogenous, moderate to marked enhancement on CT and MRI scans and a characteristic whirling or band-like pattern on T2-weighted images. DWI can be useful for the differentiation of desmoid tumors from malignant soft tissue tumors.
Keyword
MeSH Terms
Figure
Reference
-
1. Azizi L, Balu M, Belkacem A, Lewin M, Tubiana JM, Arrive L. MRI features of mesenteric desmoid tumors in familial adenomatous polyposis. AJR Am J Roentgenol. 2005; 184:1128–1135.2. Shinagare AB, Ramaiya NH, Jagannathan JP, et al. A to Z of desmoid tumors. AJR Am J Roentgenol. 2011; 197:W1008–W1014.3. de Bree E, Keus R, Melissas J, Tsiftsis D, van Coevorden F. Desmoid tumors: need for an individualized approach. Expert Rev Anticancer Ther. 2009; 9:525–535.4. Healy JC, Reznek RH, Clark SK, Phillips RK, Armstrong P. MR appearances of desmoid tumors in familial adenomatous polyposis. AJR Am J Roentgenol. 1997; 169:465–472.5. Dinauer PA, Brixey CJ, Moncur JT, Fanburg-Smith JC, Murphey MD. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics. 2007; 27:173–187.6. Lee JC, Thomas JM, Phillips S, Fisher C, Moskovic E. Aggressive fibromatosis: MRI features with pathologic correlation. AJR Am J Roentgenol. 2006; 186:247–254.7. Liu P, Thorner P. MRI of fibromatosis: with pathologic correlation. Pediatr Radiol. 1992; 22:587–589.8. Casillas J, Sais GJ, Greve JL, Iparraguirre MC, Morillo G. Imaging of intra- and extraabdominal desmoid tumors. Radiographics. 1991; 11:959–968.9. Walker EA, Petscavage JM, Brian PL, Logie CI, Montini KM, Murphey MD. Imaging features of superficial and deep fibromatoses in the adult population. Sarcoma. 2012; 2012:215810.10. Hayashi K, Takamura M, Yokoyama H, et al. A mesenteric desmoid tumor with rapid progression. Intern Med. 2017; 56:505–508.11. Shields CJ, Winter DC, Kirwan WO, Redmond HP. Desmoid tumours. Eur J Surg Oncol. 2001; 27:701–706.12. Kransdorf MJ, Jelinek JS, Moser RP Jr, et al. Magnetic resonance appearance of fibromatosis. A report of 14 cases and review of the literature. Skeletal Radiol. 1990; 19:495–499.13. Sheth PJ, Del Moral S, Wilky BA, et al. Desmoid fibromatosis: MRI features of response to systemic therapy. Skeletal Radiol. 2016; 45:1365–1373.14. Vandevenne JE, De Schepper AM, De Beuckeleer L, et al. New concepts in understanding evolution of desmoid tumors: MR imaging of 30 lesions. Eur Radiol. 1997; 7:1013–1019.15. Oka K, Yakushiji T, Sato H, et al. Usefulness of diffusion-weighted imaging for differentiating between desmoid tumors and malignant soft tissue tumors. J Magn Reson Imaging. 2011; 33:189–193.16. Hartman TE, Berquist TH, Fetsch JF. MR imaging of extraabdominal desmoids: differentiation from other neoplasms. AJR Am J Roentgenol. 1992; 158:581–585.17. Xu H, Koo HJ, Lim S, et al. Desmoid-type fibromatosis of the thorax: CT, MRI, and FDG PET characteristics in a large series from a tertiary referral center. Medicine (Baltimore). 2015; 94:e154.18. Einstein DM, Tagliabue JR, Desai RK. Abdominal desmoids: CT findings in 25 patients. AJR Am J Roentgenol. 1991; 157:275–279.19. Sundaram M, McGuire MH, Schajowicz F. Soft-tissue masses: histologic basis for decreased signal (short T2) on T2-weighted MR images. AJR Am J Roentgenol. 1987; 148:1247–1250.20. Braschi-Amirfarzan M, Keraliya AR, Krajewski KM, et al. Role of imaging in management of desmoid-type fibromatosis: a primer for radiologists. Radiographics. 2016; 36:767–782.21. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986; 161:401–407.22. Manenti G, Di Roma M, Mancino S, et al. Malignant renal neoplasms: correlation between ADC values and cellularity in diffusion weighted magnetic resonance imaging at 3 T. Radiol Med. 2008; 113:199–213.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intrathoracic Desmoid Tumor: A Case Report and Radiological Evaluation
- Desmoid Tumor of the Facet Joint: A Case Report
- Desmoid Tumor in Familial Adenomatous Polyposis (FAP)
- The Evaluation of Magnetic Resonance Imaging Application in Treatment of Tumors in Orthopedics
- Progression of Desmoid Tumors in Familial Polyposis: A Case Report