J Pathol Transl Med.
2017 May;51(3):306-313. 10.4132/jptm.2017.01.19.
Overexpression of POSTN in Tumor Stroma Is a Poor Prognostic Indicator of Colorectal Cancer
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. ghkang@snu.ac.kr
- 2Laboratory of Epigenetics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, SMG-SNU Boramae Medical Center, Seoul, Korea.
- KMID: 2392597
- DOI: http://doi.org/10.4132/jptm.2017.01.19
Abstract
- BACKGROUND
Tumor microenvironment has recently drawn attention in that it is related with tumor prognosis. Cancer-associated fibroblast also plays a critical role in cancer invasiveness and progression in colorectal cancers. Periostin (POSTN), originally identified to be expressed in osteoblasts and osteoblast-derived cells, is expressed in cancer-associated fibroblasts in several tissue types of cancer. Recent studies suggest an association between stromal overexpression of POSTN and poor prognosis of cancer patients.
METHODS
We analyzed colorectal cancer cases for their expression status of POSTN in tumor stroma using immunohistochemistry and correlated the expression status with clinicopathological and molecular features.
RESULTS
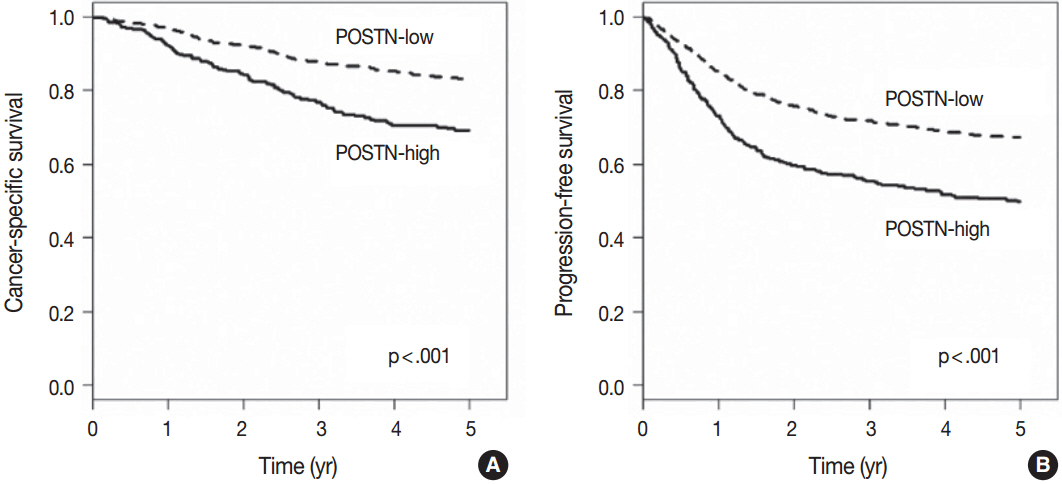
High level of POSTN expression in tumor stroma was closely associated with tumor location in proximal colon, infiltrative growth pattern, undifferentiated histology, tumor budding, luminal necrosis, and higher TNM stage. High expression status of POSTN in tumor stroma was found to be an independent prognostic parameter implicating poor 5-year cancer-specific survival and 5-year progression-free survival.
CONCLUSIONS
Our findings suggest that POSTN overexpression in tumor stroma of colorectal cancers could be a possible candidate marker for predicting poor prognosis in patients with colorectal cancers.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Multiplicity of Advanced T Category–Tumors Is a Risk Factor for Survival in Patients with Colorectal Carcinoma
Hye Eun Park, Seungyeon Yoo, Jeong Mo Bae, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang
J Pathol Transl Med. 2018;52(6):386-395. doi: 10.4132/jptm.2018.10.02.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. O’Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008; 26:2336–41.3. Bozóky B, Savchenko A, Csermely P, et al. Novel signatures of cancer-associated fibroblasts. Int J Cancer. 2013; 133:286–93.
Article4. Chen WJ, Ho CC, Chang YL, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014; 5:3472.
Article5. Huang L, Xu AM, Liu S, Liu W, Li TJ. Cancer-associated fibroblasts in digestive tumors. World J Gastroenterol. 2014; 20:17804–18.
Article6. Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008; 68:918–26.
Article7. Pankova D, Chen Y, Terajima M, et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol Cancer Res. 2016; 14:287–95.
Article8. Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011; 459:465–75.
Article9. Kikuchi Y, Kashima TG, Nishiyama T, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 2008; 56:753–64.
Article10. Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol. 2008; 21:1044–53.
Article11. Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem. 2006; 281:19700–8.
Article12. Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and αVβ5 integrins and promotes cell motility. Cancer Res. 2002; 62:5358–64.13. Baril P, Gangeswaran R, Mahon PC, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007; 26:2082–94.14. Bao S, Ouyang G, Bai X, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004; 5:329–39.
Article15. Sung PL, Jan YH, Lin SC, et al. Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma. Oncotarget. 2016; 7:4036–47.
Article16. Underwood TJ, Hayden AL, Derouet M, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol. 2015; 235:466–77.
Article17. Xu X, Chang W, Yuan J, et al. Periostin expression in intra-tumoral stromal cells is prognostic and predictive for colorectal carcinoma via creating a cancer-supportive niche. Oncotarget. 2016; 7:798–813.18. Kim JH, Shin SH, Kwon HJ, Cho NY, Kang GH. Prognostic implications of CpG island hypermethylator phenotype in colorectal cancers. Virchows Arch. 2009; 455:485–94.
Article19. Ratajczak-Wielgomas K, Dziegiel P. The role of periostin in neoplastic processes. Folia Histochem Cytobiol. 2015; 53:120–32.
Article20. Kyutoku M, Taniyama Y, Katsuragi N, et al. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011; 28:181–6.
Article21. Shao R, Bao S, Bai X, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004; 24:3992–4003.
Article22. Takanami I, Abiko T, Koizumi S. Expression of periostin in patients with non-small cell lung cancer: correlation with angiogenesis and lymphangiogenesis. Int J Biol Markers. 2008; 23:182–6.
Article23. Wu G, Wang X, Zhang X. Clinical implications of periostin in the liver metastasis of colorectal cancer. Cancer Biother Radiopharm. 2013; 28:298–302.
Article24. Xiao ZM, Wang XY, Wang AM. Periostin induces chemoresistance in colon cancer cells through activation of the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem. 2015; 62:401–6.
Article25. Dawson H, Lugli A. Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front Med (Lausanne). 2015; 2:11.
Article26. Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015; 527:525–30.
Article27. Koelzer VH, Dawson H, Andersson E, et al. Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Transl Res. 2015; 166:207–17.
Article28. Ueno H, Shinto E, Shimazaki H, et al. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol. 2015; 22:1504–12.
Article29. De Sousa EM, Wang X, Jansen M, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013; 19:614–8.
Article30. Fessler E, Drost J, van Hooff SR, et al. TGFbeta signaling directs serrated adenomas to the mesenchymal colorectal cancer subtype. EMBO Mol Med. 2016; 8:745–60.31. Kim JH, Bae JM, Cho NY, Kang GH. Distinct features between MLH1-methylated and unmethylated colorectal carcinomas with the CpG island methylator phenotype: implications in the serrated neoplasia pathway. Oncotarget. 2016; 7:14095–111.
Article32. Tsai JH, Cheng CH, Chen CC, et al. Traditional serrated adenoma with BRAF mutation is associated with synchronous/metachronous BRAF-mutated serrated lesions. Histopathology. 2016; 68:810–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Study of p53 Expression and DNA Ploidy in Colorectal Carcinoma
- The Stromal Overexpression of Decay Accelerating Factor (DAF/CD55) Correlates with Poor Clinical Outcome in Colorectal Cancer Patients
- Significance of p53 Overexpression in the Metastasis of Colorectal Cancer
- Relation Between p53 Overexpression and Extablished Prognostic Facors in Colorectal Cancer
- Clinical Significance of p53 and Ki-67 Expression in Colorectal Cancer