J Pathol Transl Med.
2017 Jul;51(4):428-432. 10.4132/jptm.2016.09.16.
Perivascular Epithelioid Cell Tumor in the Stomach
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul, Korea. woohokim@snu.ac.kr
- KMID: 2392585
- DOI: http://doi.org/10.4132/jptm.2016.09.16
Abstract
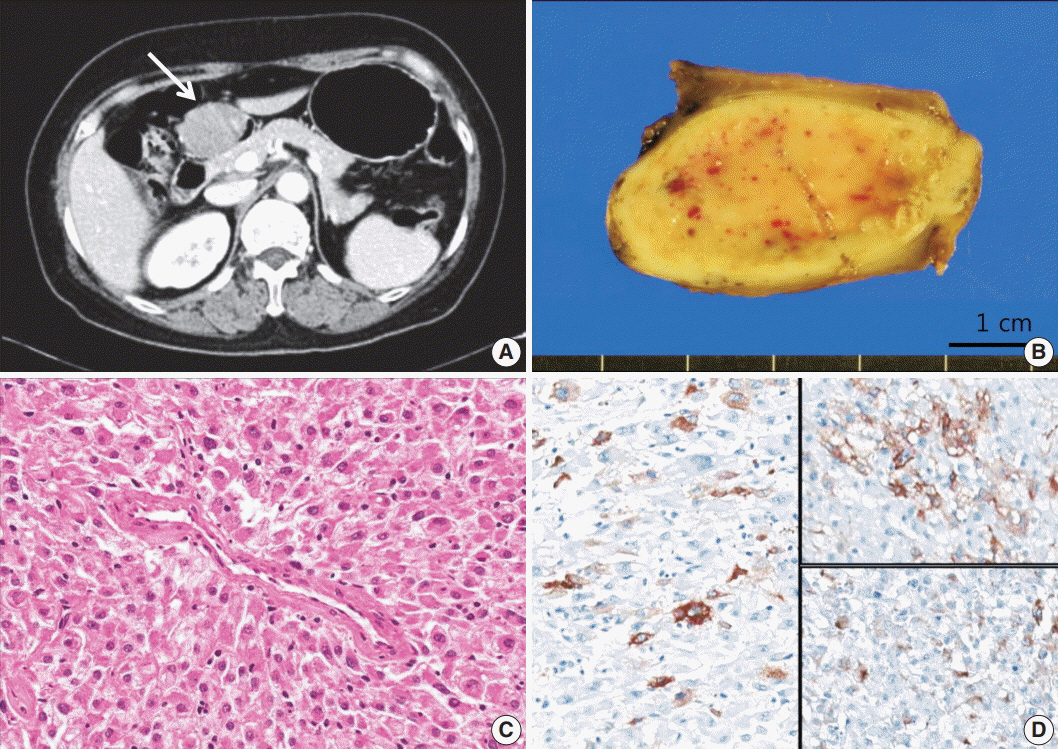
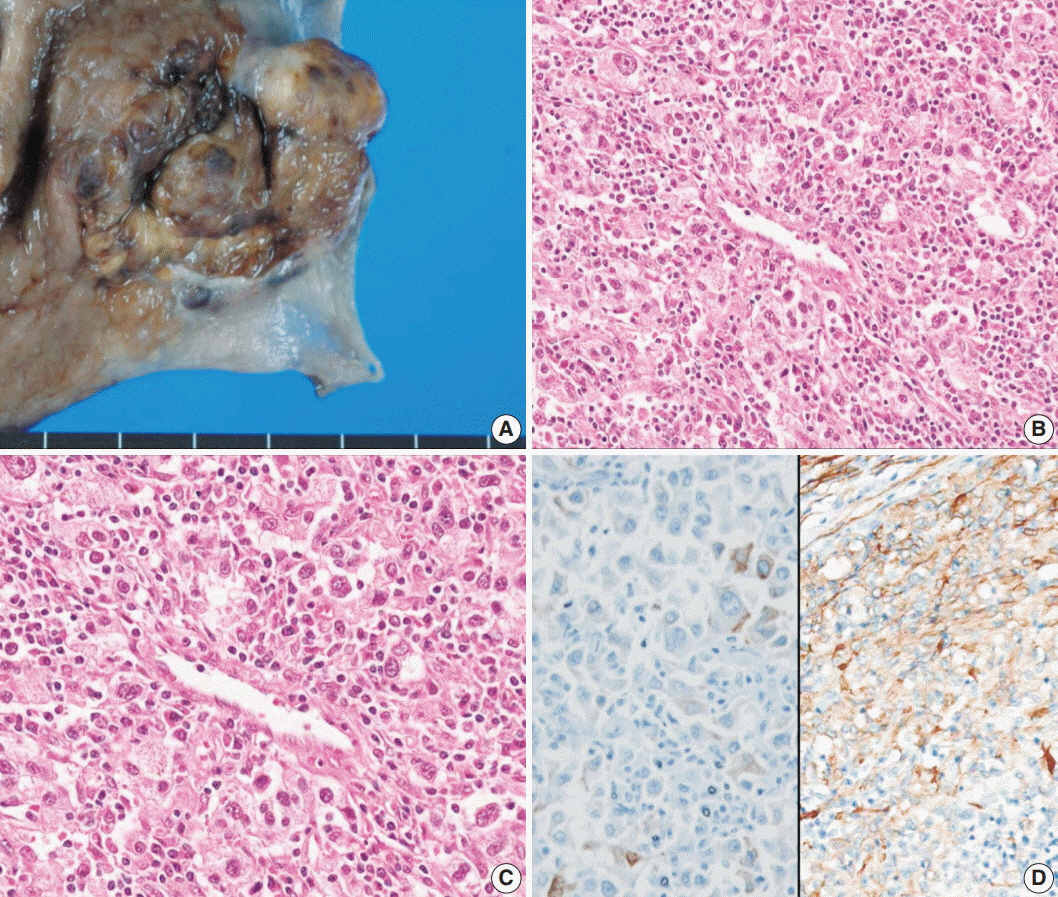
- Perivascular epithelioid cell tumors or PEComas can arise in any location in the body. However, a limited number of cases of gastric PEComa have been reported. We present two cases of gastric PEComas. The first case involved a 62-year-old woman who presented with a 4.2 cm gastric subepithelial mass in the prepyloric antrum, and the second case involved a 67-year-old man with a 5.0 cm mass slightly below the gastroesophageal junction. Microscopic examination revealed that both tumors were composed of perivascular epithelioid cells that were immunoreactive for melanocytic and smooth muscle markers. Prior to surgery, the clinical impression of both tumors was gastrointestinal stromal tumor (GIST), and the second case was erroneously diagnosed as GIST even after microscopic examination. Although gastric PEComa is a very rare neoplasm, it should be considered in the differential diagnosis of gastric submucosal lesions.
Keyword
MeSH Terms
Figure
Reference
-
1. Pea M, Bonetti F, Zamboni G, et al. Melanocyte-marker-HMB-45 is regularly expressed in angiomyolipoma of the kidney. Pathology. 1991; 23:185–8.
Article2. Adema GJ, de Boer AJ, Vogel AM, Loenen WA, Figdor CG. Molecular characterization of the melanocyte lineage-specific antigen gp100. J Biol Chem. 1994; 269:20126–33.
Article3. Pea M, Bonetti F, Zamboni G, Martignoni G, Fiore-Donati L, Doglioni C. Clear cell tumor and angiomyolipoma. Am J Surg Pathol. 1991; 15:199–202.
Article4. Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992; 16:307–8.
Article5. Zamboni G, Pea M, Martignoni G, et al. Clear cell “sugar” tumor of the pancreas: a novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996; 20:722–30.6. Hornick JL, Pan CC. PEComa. In : Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F, editors. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press;2013. p. 230–1.7. Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg Pathol. 2000; 24:1239–46.8. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005; 29:1558–75.9. Doyle LA, Hornick JL, Fletcher CD. PEComa of the gastrointestinal tract: clinicopathologic study of 35 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2013; 37:1769–82.10. Im S, Yoo C, Jung JH, Choi HJ, Yoo J, Kang CS. Primary perivascular epithelioid cell tumor in the rectum: a case report and review of the literature. Pathol Res Pract. 2013; 209:244–8.
Article11. Shi HY, Wei LX, Sun L, Guo AT. Clinicopathologic analysis of 4 perivascular epithelioid cell tumors (PEComas) of the gastrointestinal tract. Int J Surg Pathol. 2010; 18:243–7.
Article12. Mitteldorf CA, Birolini D, da Camara-Lopes LH. A perivascular epithelioid cell tumor of the stomach: an unsuspected diagnosis. World J Gastroenterol. 2010; 16:522–5.
Article13. Waters PS, Mitchell DP, Murphy R, McKenna M, Waldron RP. Primary malignant gastric PEComa: diagnostic and technical dilemmas. Int J Surg Case Rep. 2012; 3:89–91.14. Yamada S, Nabeshima A, Noguchi H, et al. Coincidence between malignant perivascular epithelioid cell tumor arising in the gastric serosa and lung adenocarcinoma. World J Gastroenterol. 2015; 21:1349–56.
Article15. Kumar M, Kumar V, Abrina V, Kaur S, Kumar A, Maroules M. Malignant gastric PEComa: a rare malignancy. Am J Cancer Case Rep. 2015; 3:209–14.16. Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010; 41:1–15.
Article17. Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006; 48:75–82.
Article18. Argani P, Aulmann S, Illei PB, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010; 34:1395–406.19. Lee HE, Kim MA, Lee HS, Lee BL, Kim WH. Characteristics of KIT-negative gastrointestinal stromal tumours and diagnostic utility of protein kinase C theta immunostaining. J Clin Pathol. 2008; 61:722–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Perivascular Epithelioid Cell Tumor (PEComa) of the Liver: A Case Report and Review of the Literature
- A Case of Primary Cutaneous Perivascular Epithelioid Cell Tumor
- A case of perivascular epithelioid cell tumor of the uterus
- Pigmented Perivascular Epithelioid Cell Tumor (PEComa) of the Kidney: A Case Report and Review of the Literature
- Malignant Perivascular Epithelioid Cell Tumor (PEComa) Arising in the Omentum with Metastatic Peritoneal Seeding: A Case Report