J Pathol Transl Med.
2017 Jul;51(4):352-358. 10.4132/jptm.2017.03.15.
Epstein-Barr Virus–Associated Lymphoproliferative Disorders: Review and Update on 2016 WHO Classification
- Affiliations
-
- 1Department of Pathology, Inje University, Sanggye Paik Hospital, Seoul, Korea.
- 2Sungkyunkwan University, School of Medicine, Samsung Medical Center, Seoul, Korea.
- 3SMG-SNU Boramae Medical Center, Seoul National University, Seoul, Korea.
- 4Korea Cancer Center Hospital, Seoul, Korea.
- 5Eulji University Hospital, Eulji University School of Medicine, Daejeon, Korea.
- 6Gangnam St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 7Seoul National University Bundang Hospital, Seongnam, Korea.
- 8Ulsan University Hospital, Ulsan University School of Medicine, Ulsan, Korea.
- 9Chonnam National University Hospital, Chonnam National University, Gwangju, Korea.
- 10Ajou University Hospital, Suwon, Korea.
- 11Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea. Jrhuh@amc.seoul.kr
- KMID: 2392574
- DOI: http://doi.org/10.4132/jptm.2017.03.15
Abstract
- Epstein-Barr virus (human herpesvirus-4) is very common virus that can be detected in more than 95% of the human population. Most people are asymptomatic and live their entire lives in a chronically infected state (IgG positive). However, in some populations, the Epstein-Barr virus (EBV) has been involved in the occurrence of a wide range of B-cell lymphoproliferative disorders (LPDs), including Burkitt lymphoma, classic Hodgkin's lymphoma, and immune-deficiency associated LPDs (post-transplant and human immunodeficiency virus-associated LPDs). T-cell LPDs have been reported to be associated with EBV with a subset of peripheral T-cell lymphomas, angioimmunoblastic T-cell lymphomas, extranodal nasal natural killer/T-cell lymphomas, and other rare histotypes. This article reviews the current evidence covering EBV-associated LPDs based on the 2016 classification of the World Health Organization. These LPD entities often pose diagnostic challenges, both clinically and pathologically, so it is important to understand their unique pathophysiology for correct diagnoses and optimal management.
MeSH Terms
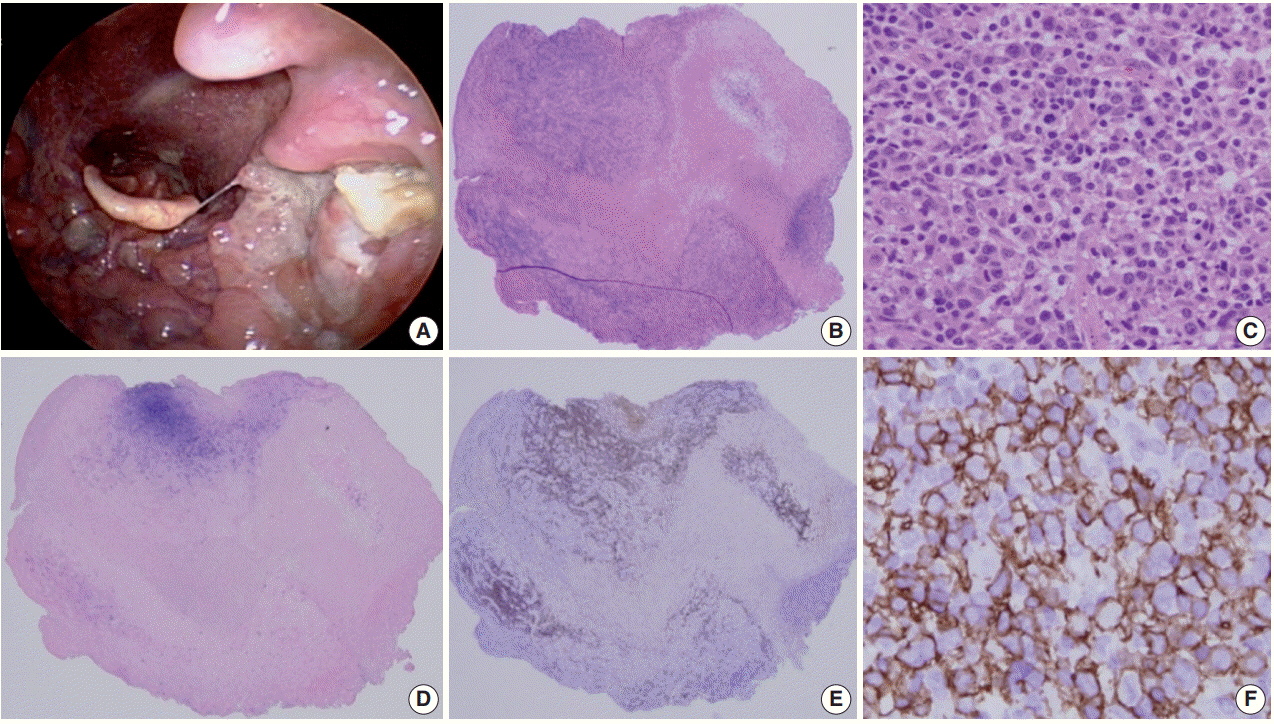
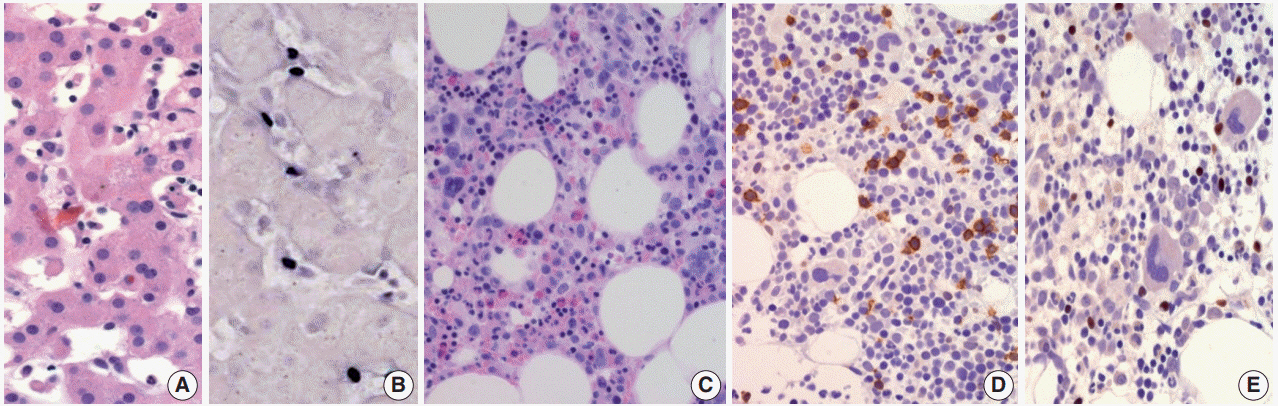
Figure
Cited by 1 articles
-
Diffuse Large B-Cell Lymphoma Arising within Ileal Neobladder: An Expanding Spectrum of Diffuse Large B-Cell Lymphoma Associated with Chronic Inflammation
Hyekyung Lee, Hyunbin Shin, Nae Yu Kim, Hyun Sik Park, Jinsung Park
Cancer Res Treat. 2019;51(4):1666-1670. doi: 10.4143/crt.2019.022.
Reference
-
1. Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000; 343:481–92.
Article2. Pittaluga S, Said J. Virally associated B cell lymphoproliferative disease. In : Jaffe E, Arbor DA, Campo E, Harris NL, Quintanilla-Fend L, editors. Hematopathology. 2nd ed. Philadelphia: Elsevier Heealth Science;2016. p. 547–607.3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–90.
Article4. Chaganti S, Heath EM, Bergler W, et al. Epstein-Barr virus colonization of tonsillar and peripheral blood B-cell subsets in primary infection and persistence. Blood. 2009; 113:6372–81.
Article5. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012; 19:14–20.
Article6. Fletcher C. Diagnostic histopathology of tumors. 3rd ed. Philadelphia: Churchill Livingstone/Elsevier;2007. p. 1260–1.7. Lekstrom-Himes JA, Dale JK, Kingma DW, Diaz PS, Jaffe ES, Straus SE. Periodic illness associated with Epstein-Barr virus infection. Clin Infect Dis. 1996; 22:22–7.8. Cho EY, Kim KH, Kim WS, Yoo KH, Koo HH, Ko YH. The spectrum of Epstein-Barr virus-associated lymphoproliferative disease in Korea: incidence of disease entities by age groups. J Korean Med Sci. 2008; 23:185–92.
Article9. Cohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011; 117:5835–49.
Article10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumors of hematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press;2008.11. Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015; 126:863–72.
Article12. Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010; 34:405–17.
Article13. Hongyo T, Kurooka M, Taniguchi E, et al. Frequent p53 mutations at dipyrimidine sites in patients with pyothorax-associated lymphoma. Cancer Res. 1998; 58:1105–7.14. Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979; 43:360–73.15. Song JY, Pittaluga S, Dunleavy K, et al. Lymphomatoid granulomatosis: a single institute experience: pathologic findings and clinical correlations. Am J Surg Pathol. 2015; 39:141–56.16. Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012; 119:673–86.
Article17. Toga A, Wada T, Sakakibara Y, et al. Clinical significance of cloned expansion and CD5 down-regulation in Epstein-Barr Virus (EBV)-infected CD8+ T lymphocytes in EBV-associated hemophagocytic lymphohistiocytosis. J Infect Dis. 2010; 201:1923–32.18. Ohshima K, Kimura H, Yoshino T, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. 2008; 58:209–17.
Article19. Ko YH, Kim HJ, Oh YH, et al. EBV-associated T and NK cell lymphoproliferative disorders: consensus report of the 4th Asian Hematopathology Workshop. J Hematopathol. 2012; 5:319–24.
Article20. Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009; 20:1472–82.
Article21. Park S, Ko YH. Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorders. J Dermatol. 2014; 41:29–39.
Article22. Miyake T, Yamamoto T, Hirai Y, et al. Survival rates and prognostic factors of Epstein-Barr virus-associated hydroa vacciniforme and hypersensitivity to mosquito bites. BrJ Dermatol. 2015; 172:56–63.
Article23. Cho KH, Kim CW, Heo DS, et al. Epstein-Barr virus-associated peripheral T-cell lymphoma in adults with hydroa vacciniforme-like lesions. Clin Exp Dermatol. 2001; 26:242–7.
Article24. Kumar S, et al. FulminantEBV(+)T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000; 96:443–51.25. Hong M, Lee T, Kang SY, Kim SJ, Kim W, Ko YH. Nasal-type NK/T-cell lymphomas are more frequently T rather than NK lineage based on T-cell receptor gene, RNA, and protein studies: lineage does not predict clinical behavior. Mod Pathol. 2016; 29:430–43.
Article26. Chan JK, Sin VC, Wong KF, et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood. 1997; 89:4501–13.
Article27. Kato S, Asano N, Miyata-Takata T, et al. T-cell receptor (TCR) phenotype of nodal Epstein-Barr virus (EBV)-positive cytotoxic T-cell lymphoma (CTL): a clinicopathologic study of 39 cases. Am J Surg Pathol. 2015; 39:462–71.28. Kim JH, Sung JY, Han JH, KoYH; Hematopathology Study Group of the Korean Society of Pathologists. Epstein-Barr virus-positive nodal T/NK-cell lymphoma: an analysis of 15 cases with distinct clinicopathological features. Hum Pathol. 2015; 46:981–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorders
- Epstein Barr Virus-associated Vesicular Eruptions of the Face
- An Adult Case of Severe Chronic Active Epstein-Barr Virus Infection with T-Cell Lymphoproliferative Disorder
- EBV-Associated Lymphoproliferative Disorders
- Epstein-Barr Virus Related Polymorphic Posttransplantation Lymphoproliferative Disease in a Patient with Latent Infection of JC Virus