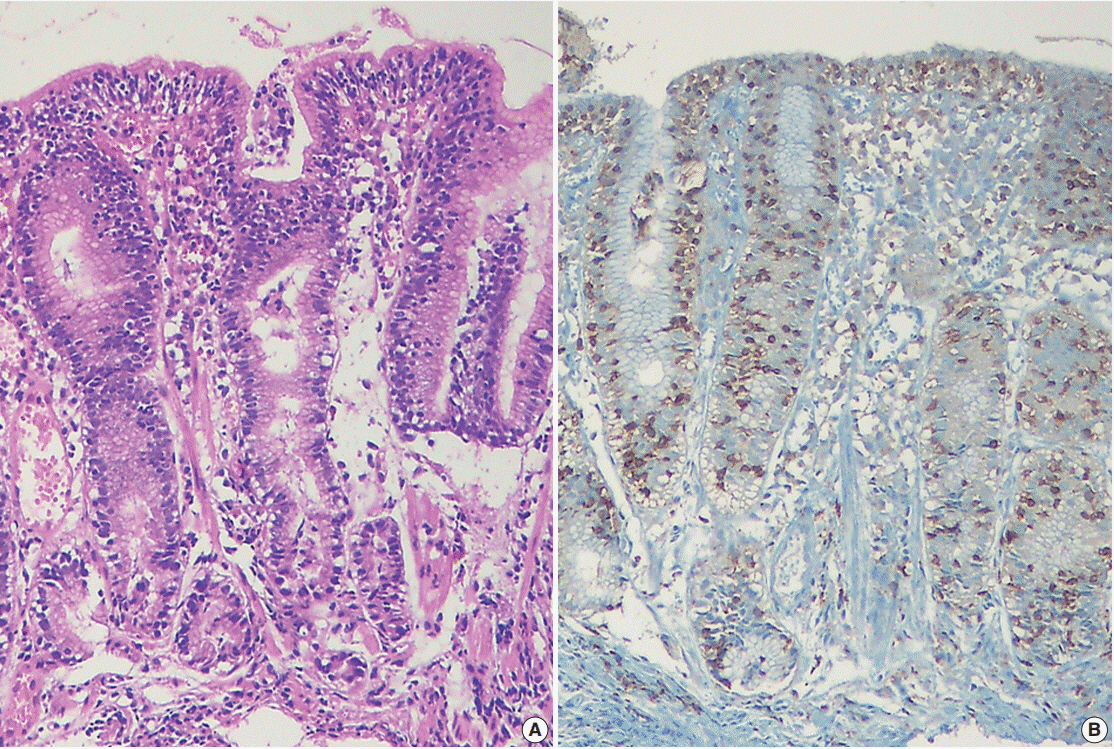
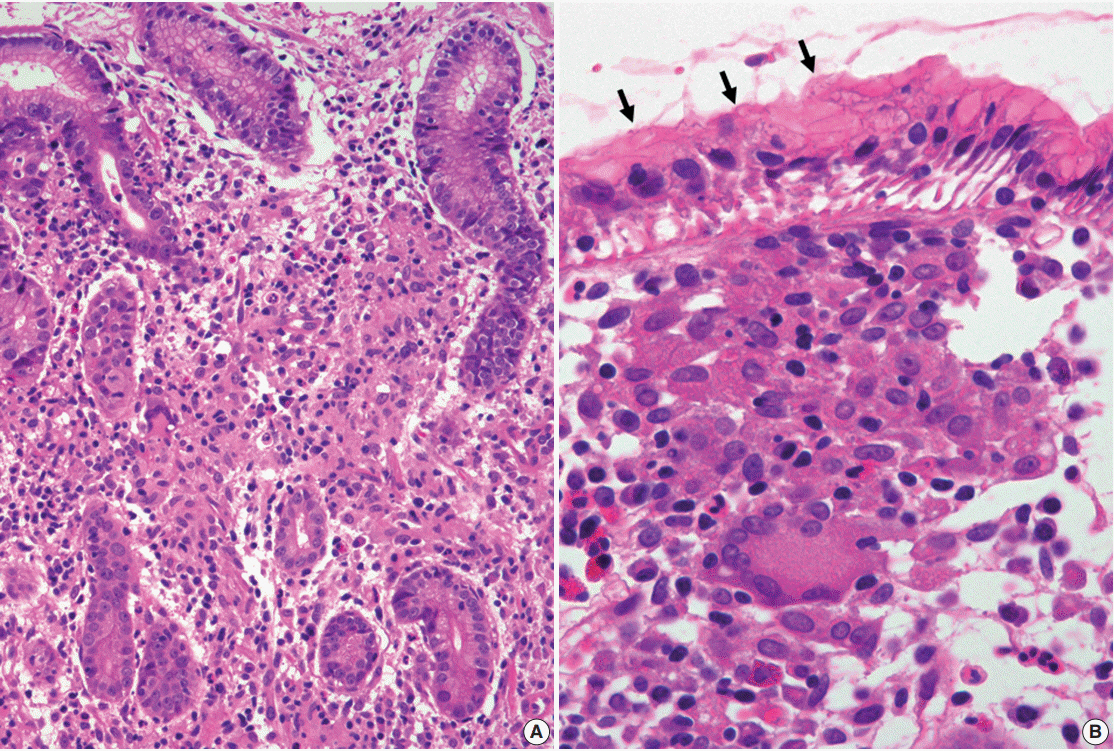
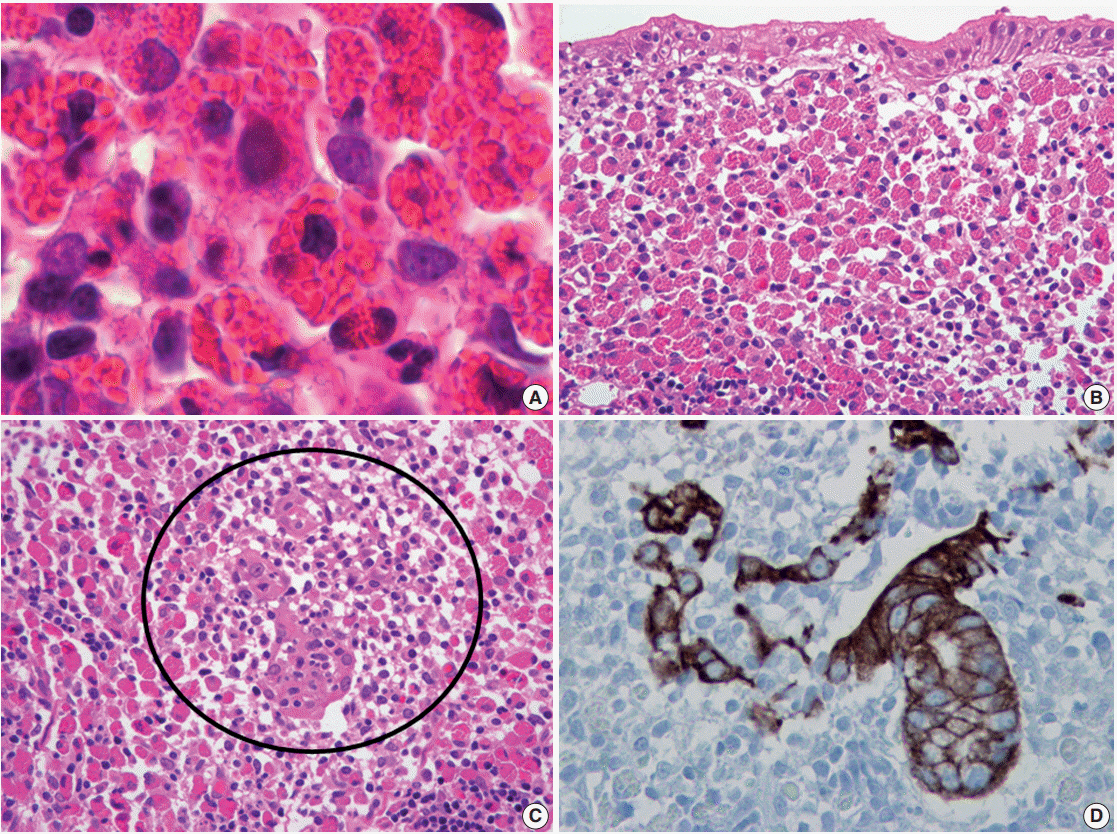
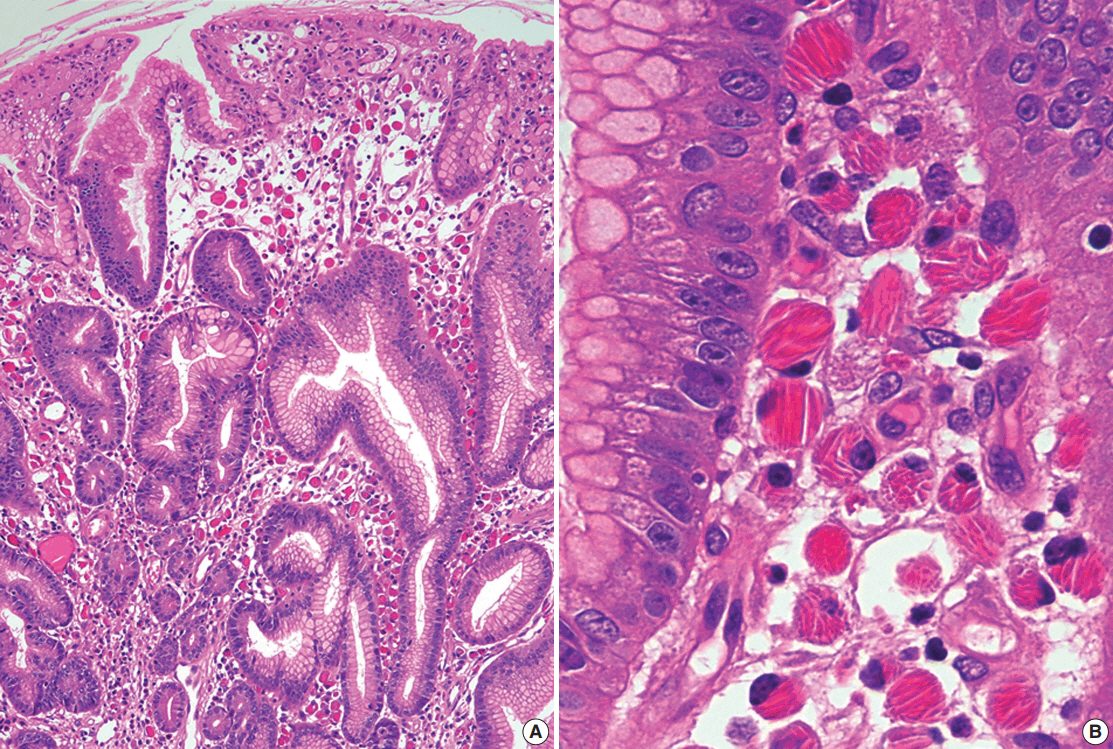
J Pathol Transl Med.
2017 Jul;51(4):341-351. 10.4132/jptm.2017.04.03.
Rare Gastric Lesions Associated with Helicobacter pylori Infection: A Histopathological Review
- Affiliations
-
- 1Department of Pathology, Inje University Ilsan Paik Hospital, Goyang, Korea.
- KMID: 2392573
- DOI: http://doi.org/10.4132/jptm.2017.04.03
Abstract
- Helicobacter pylori infection is associated with chronic gastritis, peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma. However, some rare gastric lesions exhibiting distinctive histological features may also be associated with H. pylori infection, including lymphocytic gastritis, granulomatous gastritis, Russell body gastritis, or crystal-storing histiocytosis. Although diverse factors can contribute to their development, there is convincing evidence that H. pylori infection may play a pathogenic role. These findings are mainly based on studies in patients with these lesions who exhibited clinical and histological improvements after H. pylori eradication therapy. Thus, H. pylori eradication therapy might be indicated in patients with no other underlying disease, particularly in countries with a high prevalence of H. pylori infection. This review describes the characteristic histological features of these rare lesions and evaluates the evidence regarding a causative role for H. pylori infection in their pathogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Dixon MF, Wyatt JI, Burke DA, Rathbone BJ. Lymphocytic gastritis- -relationship to Campylobacter pylori infection. J Pathol. 1988; 154:125–32.2. Carmack SW, Lash RH, Gulizia JM, Genta RM. Lymphocytic disorders of the gastrointestinal tract: a review forthe practicing pathologist. AdvAnat Pathol. 2009; 16:290–309.3. Genta RM, Lash RH. Helicobacter pylori-negative gastritis: seek, yet ye shall not always find. Am J Surg Pathol. 2010; 34:e25–34.4. Srivastava A, Lauwers GY. Pathology of non-infective gastritis. Histopathology. 2007; 50:15–29.
Article5. Wu TT, Hamilton SR. Lymphocytic gastritis: association with etiology and topology. Am J Surg Pathol. 1999; 23:153–8.
Article6. Ectors NL, Dixon MF, Geboes KJ, Rutgeerts PJ, Desmet VJ, Vantrappen GR. Granulomatous gastritis: a morphological and diagnostic approach. Histopathology. 1993; 23:55–61.
Article7. Maeng L, Choi K, Kang CS, Kim KM. Granulomatous gastritis: a clinicopathologic analysis of 18 biopsy cases. Am J Surg Pathol. 2004; 28:941–5.8. Paik S, Kim SH, Kim JH, Yang WI, Lee YC. Russell body gastritis associated with Helicobacter pylori infection: a case report. J Clin Pathol. 2006; 59:1316–9.9. Zhang H, Jin Z, Cui R. Russell body gastritis/duodenitis: a case series and description of immunoglobulin light chain restriction. Clin Res Hepatol Gastroenterol. 2014; 38:e89–97.
Article10. Stewart CJ, Spagnolo DV. Crystalline plasma cell inclusions in helicobacter-associated gastritis. J Clin Pathol. 2006; 59:851–4.
Article11. Joo M, Kwak JE, Chang SH, et al. Localized gastric crystal-storing histiocytosis. Histopathology. 2007; 51:116–9.
Article12. Renault M, Subramony C, Hood B, Bishop P, Nowicki M. Age-related differences in granulomatous gastritis: a retrospective, clinicopathological analysis. J Clin Pathol. 2010; 63:347–50.
Article13. Hayat M, Arora DS, Dixon MF, Clark B, O’Mahony S. Effects of Helicobacter pylori eradication on the natural history of lymphocytic gastritis. Gut. 1999; 45:495–8.14. Madisch A, Miehlke S, Neuber F, et al. Healing of lymphocytic gastritis after Helicobacter pylori eradication therapy--a randomized, double-blind, placebo-controlled multicentre trial. Aliment Pharmacol Ther. 2006; 23:473–9.15. Müller H, Volkholz H, Stolte M. Healing of lymphocytic gastritis by eradication of Helicobacter pylori. Digestion. 2001; 63:14–9.16. Niemelä S, Karttunen T, Kerola T, Karttunen R. Ten year follow up study of lymphocytic gastritis: further evidence on Helicobacter pylori as a cause of lymphocytic gastritis and corpus gastritis. J Clin Pathol. 1995; 48:1111–6.17. Miyamoto M, Haruma K, Yoshihara M, et al. Isolated granulomatous gastritis successfully treated by Helicobacter pylori eradication: a possible association between granulomatous gastritis and Helicobacter pylori. J Gastroenterol. 2003; 38:371–5.18. Ozturk Y, Buyukgebiz B, Ozer E, Arslan N, Bekem O, Hizli S. Resolution of Helicobacter pylori associated granulomatous gastritis in a child after eradication therapy. J Pediatr Gastroenterol Nutr. 2004; 39:286–7.19. Tazawa K, Tsutsumi Y. Localized accumulation of Russell bodycontaining plasma cells in gastric mucosa with Helicobacter pylori infection: ‘Russell body gastritis’. Pathol Int. 1998; 48:242–4.20. Wolkersdörfer GW, Haase M, Morgner A, Baretton G, Miehlke S. Monoclonal gammopathy of undetermined significance and Russell body formation in Helicobacter pylori gastritis. Helicobacter. 2006; 11:506–10.21. Yoon JB, Lee TY, Lee JS, et al. Two cases of Russell body gastritis treated by Helicobacter pylori eradication. Clin Endosc. 2012; 45:412–6.22. Dhillon AP, Sawyerr A. Granulomatous gastritis associated with Campylobacter pylori. APMIS. 1989; 97:723–7.
Article23. Delgado JS, Landa E, Ben-Dor D. Granulomatous gastritis and Helicobacter pylori infection. Isr MedAssoc J. 2013; 15:317–8.24. Koyama S, Nagashima F. Idiopathic granulomatous gastritis with multiple aphthoid ulcers. Intern Med. 2003; 42:691–5.
Article25. Haot J, Hamichi L, Wallez L, Mainguet P. Lymphocytic gastritis: a newly described entity: a retrospective endoscopic and histological study. Gut. 1988; 29:1258–64.
Article26. Haot J, Jouret A, Willette M, Gossuin A, Mainguet P. Lymphocytic gastritis: prospective study of its relationship with varioliform gastritis. Gut. 1990; 31:282–5.27. Nielsen JA, Roberts CA, Lager DJ, Putcha RV, Jain R, Lewin M. Lymphocytic gastritis is not associated with active Helicobacter pylori infection. Helicobacter. 2014; 19:349–55.28. Feeley KM, Heneghan MA, Stevens FM, McCarthy CF. Lymphocytic gastritis and coeliac disease: evidence of a positive association. J Clin Pathol. 1998; 51:207–10.
Article29. Alsaigh N, Odze R, Goldman H, Antonioli D, Ott MJ, Leichtner A. Gastric and esophageal intraepithelial lymphocytes in pediatric celiac disease. Am J Surg Pathol. 1996; 20:865–70.
Article30. Bhatti TR, Jatla M, Verma R, Bierly P, Russo PA, Ruchelli ED. Lymphocytic gastritis in pediatric celiac disease. Pediatr Dev Pathol. 2011; 14:280–3.
Article31. Oberhuber G, Vogelsang H, Stolte M, Muthenthaler S, Kummer JA, Radaszkiewicz T. Evidence that intestinal intraepithelial lymphocytes are activated cytotoxic T cells in celiac disease but not in giardiasis. Am J Pathol. 1996; 148:1351–7.32. Inagaki-Ohara K, Nishimura H, Sakai T, Lynch DH, Yoshikai Y. Potential for involvement of Fas antigen/Fas ligand interaction in apoptosis of epithelial cells by intraepithelial lymphocytes in murine small intestine. Lab Invest. 1997; 77:421–9.33. Han SH, Joo M, Kim KM. High proportion of granzyme B+ intraepithelial lymphocytes contributes to epithelial apoptosis in Helicobacter pylori-associated lymphocytic gastritis. Helicobacter. 2013; 18:290–8.34. Oberhuber G, Bodingbauer M, Mosberger I, Stolte M, Vogelsang H. High proportion of granzyme B-positive (activated) intraepithelial and lamina propria lymphocytes in lymphocytic gastritis. Am J Surg Pathol. 1998; 22:450–8.
Article35. Shapiro JL, Goldblum JR, Petras RE. Aclinicopathologic study of 42 patients with granulomatous gastritis. Is there really an “idiopathic” granulomatous gastritis?Am J Surg Pathol. 1996; 20:462–70.36. El Demellawy D, Otero C, Radhi J. Primary gastric lymphoma with florid granulomatous reaction. J Gastrointestin Liver Dis. 2009; 18:99–101.37. Fahimi HD, Deren JJ, Gottlieb LS, Zamcheck N. Isolated granulomatous gastritis: its relationship to disseminated sarcoidosis and regional enteritis. Gastroenterology. 1963; 45:161–75.
Article38. Sandmeier D, Bouzourene H. Does idiopathic granulomatous gastritis exist? Histopathology. 2005; 46:352–3.
Article39. Kim YS, Lee HK, Kim JO, et al. A case of H. pylori-associated granulomatous gastritis with hypertrophic gastropathy. Gut Liver. 2009; 3:137–40.40. Fuentebella J, Bass D, Longacre T, Ro K. Abdominal pain, gastrointestinal bleeding, and weight loss in a 17-year-old male. Dig Dis Sci. 2009; 54:722–4.
Article41. Gonen C, Sarioglu S, Akpinar H. Magnifying endoscopic features of granulomatous gastritis. Dig Dis Sci. 2009; 54:1602–3.
Article42. Yamane T, Uchiyama K, Ishii T, et al. Isolated granulomatous gastritis showing discoloration of lesions after Helicobacter pylori eradication. Dig Endosc. 2010; 22:140–3.43. Jones D, Bhatia VK, Krausz T, Pinkus GS. Crystal-storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999; 30:1441–8.44. Gebbers JO, Otto HF. Plasma cell alterations in ulcerative colitis: an electron microscopic study. Pathol Eur. 1976; 11:271–9.45. van den Tweel JG, Taylor CR, Parker JW, Lukes RJ. Immunoglobulin inclusions in non-Hodgkin’s lymphomas. Am J Clin Pathol. 1978; 69:306–13.
Article46. Hasegawa H. Aggregates, crystals, gels, and amyloids: intracellular and extracellular phenotypes at the crossroads of immunoglobulin physicochemical property and cell physiology. Int J Cell Biol. 2013; 2013:604867.
Article47. Valetti C, Grossi CE, Milstein C, Sitia R. Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J Cell Biol. 1991; 115:983–94.
Article48. Joo M. Gastric mucosa-associated lymphoid tissue lymphoma masquerading as Russell body gastritis. Pathol Int. 2015; 65:396–8.
Article49. Erbersdobler A, Petri S, Lock G. Russell body gastritis: an unusual, tumor-like lesion of the gastric mucosa. Arch Pathol Lab Med. 2004; 128:915–7.
Article50. Ensari A, Savas B, Okcu Heper A, Kuzu I, Idilman R. An unusual presentation of Helicobacter pylori infection: so-called “Russell body gastritis”. VirchowsArch. 2005; 446:463–6.51. Drut R, Olenchuk AB. Images in pathology: Russell body gastritis in an HIV-positive patient. Int J Surg Pathol. 2006; 14:141–2.52. Pizzolitto S, Camilot D, DeMaglio G, Falconieri G. Russell body gastritis: expanding the spectrum of Helicobacter pylori-related diseases? Pathol Res Pract. 2007; 203:457–60.53. Licci S, Sette P, Del Nonno F, Ciarletti S, Antinori A, Morelli L. Russell body gastritis associated with Helicobacter pylori infection in an HIV-positive patient: case report and review of the literature. Z Gastroenterol. 2009; 47:357–60.54. Habib C, Gang DL, Ghaoui R, Pantanowitz L. Russell body gastritis. Am J Hematol. 2010; 85:951–2.
Article55. Ushiku T, Fukayama M. Prominent Mott cell proliferation in Epstein-Barr virus-associated gastric carcinoma. Hum Pathol. 2010; 41:134–8.56. Del Gobbo A, Elli L, Braidotti P, Di Nuovo F, Bosari S, Romagnoli S. Helicobacter pylori-negative Russell body gastritis: case report. World J Gastroenterol. 2011; 17:1234–6.57. Wolf EM, Mrak K, Tschmelitsch J, Langner C. Signetring cell cancer in a patient with Russell body gastritis: a possible diagnostic pitfall. Histopathology. 2011; 58:1178–80.58. Coyne JD, Azadeh B. Russell body gastritis: a case report. Int J Surg Pathol. 2012; 20:69–70.59. Bhalla A, Mosteanu D, Gorelick S, Hani el F. Russell body gastritis in an HIV positive patient: case report and review of literature. Conn Med. 2012; 76:261–5.60. Karabagli P, Gokturk HS. Russell body gastritis: case report and review of the literature. J Gastrointestin Liver Dis. 2012; 21:97–100.61. Araki D, Sudo Y, Imamura Y, Tsutsumi Y. Russell body gastritis showing IgM kappa-type monoclonality. Pathol Int. 2013; 63:565–7.62. Soltermann A, Koetzer S, Eigenmann F, Komminoth P. Correlation of Helicobacter pylori virulence genotypes vacAand cagA with histological parameters of gastritis and patient's age. Mod Pathol. 2007; 20:878–83.63. Giron JA, Shah SL. Helicobacter pylori infection and light chain gammopathy. Clin Dev Immunol. 2013; 2013:348562.64. Lebeau A, Zeindl-Eberhart E, Müller EC, et al. Generalized crystalstoring histiocytosis associated with monoclonal gammopathy: molecular analysis of a disorder with rapid clinical course and review of the literature. Blood. 2002; 100:1817–27.
Article65. Dogan S, Barnes L, Cruz-Vetrano WP. Crystal-storing histiocytosis: report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012; 6:111–20.
Article66. Ionescu DN, Pierson DM, Qing G, Li M, Colby TV, Leslie KO. Pulmonary crystal-storing histiocytoma. Arch Pathol Lab Med. 2005; 129:1159–63.
Article67. Hirota S, Miyamoto M, Kasugai T, Kitamura Y, Morimura Y. Crystalline light-chain deposition and amyloidosis in the thyroid gland and kidneys of a patient with myeloma. Arch Pathol Lab Med. 1990; 114:429–31.68. Stirling JW, Henderson DW, Rozenbilds MA, Skinner JM, Filipic M. Crystalloidal paraprotein deposits in the cornea: an ultrastructural study of two new cases with tubular crystalloids that contain IgG kappa light chains and IgG gamma heavy chains. Ultrastruct Pathol. 1997; 21:337–44.69. Papla B, Spólnik P, Rzenno E, et al. Generalized crystal-storing histiocytosis as a presentation of multiple myeloma: a case with a possible pro-aggregation defect in the immunoglobulin heavy chain. Virchows Arch. 2004; 445:83–9.
Article70. Llobet M, Castro P, Barceló C, Trull JM, Campo E, Bernadó L. Massive crystal-storing histicytosis associated with low-grade malignant B-cell lymphoma of MALT-type of the parotid gland. Diagn Cytopathol. 1997; 17:148–52.71. Kanagal-Shamanna R, Xu-Monette ZY, Miranda RN, et al. Crystalstoring histiocytosis: a clinicopathological study of 13 cases. Histopathology. 2016; 68:482–91.
Article72. Vaid A, Caradine KD, Lai KK, Rego R. Isolated gastric crystal-storing histiocytosis: a rare marker of occult lymphoproliferative disorders. J Clin Pathol. 2014; 67:740–1.
Article73. Kapadia SB, Enzinger FM, Heffner DK, Hyams VJ, Frizzera G. Crystal-storing histiocytosis associated with lymphoplasmacytic neoplasms: report of three cases mimicking adult rhabdomyoma. Am J Surg Pathol. 1993; 17:461–7.74. Kusakabe T, Watanabe K, Mori T, Iida T, Suzuki T. Crystal-storing histiocytosis associated with MALT lymphoma of the ocular adnexa: a case report with review of literature. Virchows Arch. 2007; 450:103–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of Helicobacter pylori infection in patients of peptic ulcer among Korean people
- Two Cases of Gastric Stump Cancer: Possible Role of Helicobacter pylori Infection
- Immune Response to Helicobacter pylori Infection
- Perspective of Helicobacter pylori Research: Molecular Pathogenesis of Helicobacter pylori Virulence Factors
- The Association between Helicobacter pylori Infection and Gastric Adenocarcinoma: a Review of the Literature