Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 2Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea. cdongs@kumc.or.kr
- 3Department of Internal Medicine, Hallym University Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Kyung Hee University Hospital, Kyung Hee University School of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 6Department of Internal Medicine, Cardiovascular and Metabolic Disease Center, Inje University Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea,.
- 8Kosair Children's Hospital Research Institute, University of Louisville, Louisville, KY, USA.
- 9Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea.
- 10Department of Internal Medicine, Soon Chun Hyang University Cheonan Hospital, Soon Chun Hyang University College of Medicine, Cheonan, Korea.
- KMID: 2392481
- DOI: http://doi.org/10.4093/dmj.2017.41.5.377
Abstract
- BACKGROUND
The aim of this multicenter, randomized, double-blind study was to examine the effect of lobeglitazone, a novel thiazolidinedione, on the changes in bone mineral density (BMD) in patients with type 2 diabetes mellitus.
METHODS
A 24-week, double-blinded phase was followed by a 28-week, open-label phase, in which the placebo group also started to receive lobeglitazone. A total of 170 patients aged 34 to 76 years were randomly assigned in a 2:1 ratio to receive lobeglitazone 0.5 mg or a matching placebo orally, once daily. BMD was assessed using dual-energy X-ray absorptiometry at week 24 and at the end of the study (week 52).
RESULTS
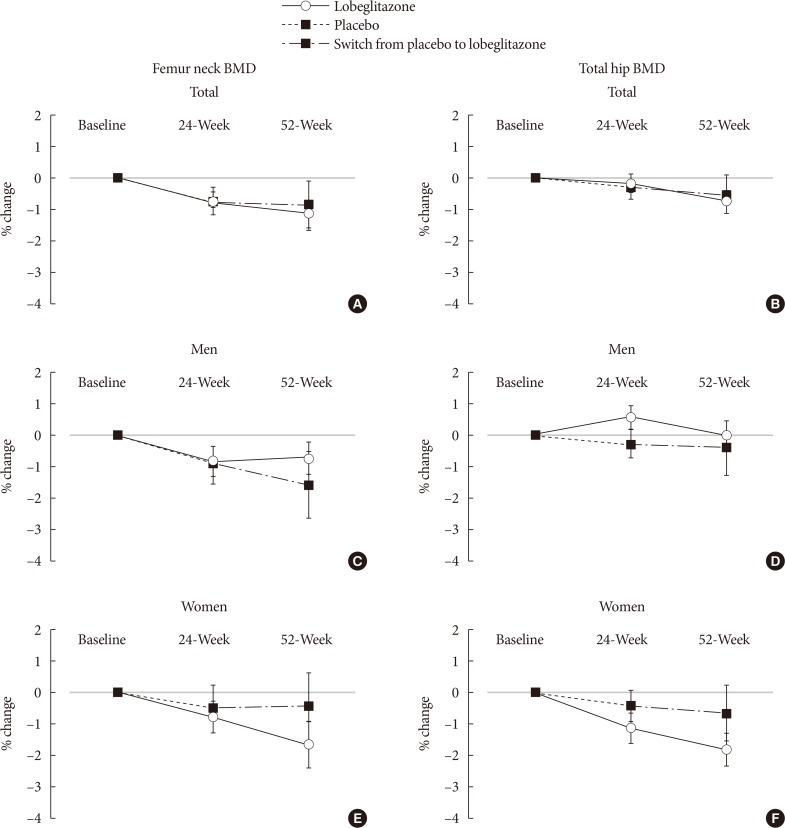
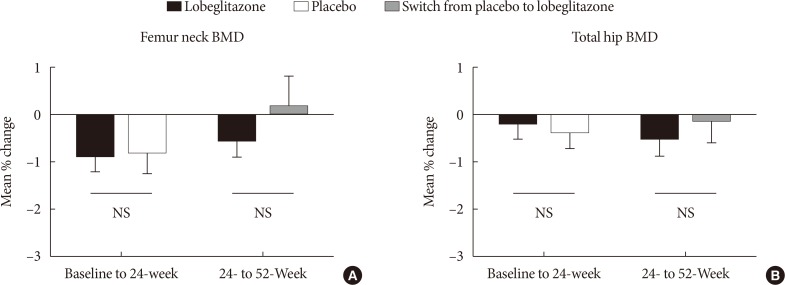
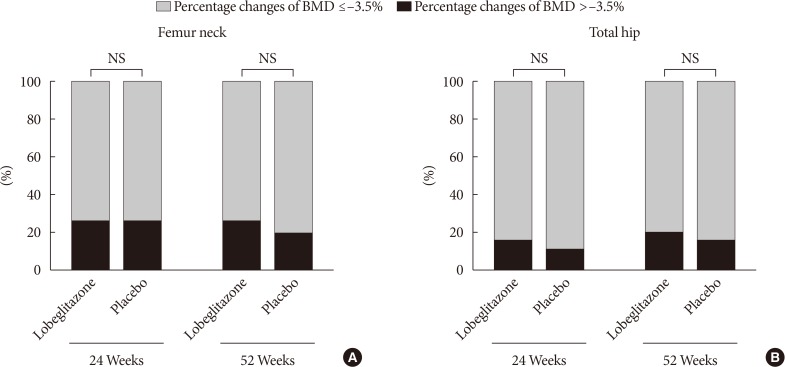
During the double-blinded phase, the femur neck BMD showed decreasing patterns in both groups, without statistical significance (−0.85%±0.36% and −0.78%±0.46% in the lobeglitazone and placebo groups, respectively). The treatment difference between the groups was 0.07%, which was also not statistically significant. Further, minimal, nonsignificant decreases were observed in both groups in the total hip BMD compared to values at baseline, and these differences also did not significantly differ between the groups. During the open-label phase, the BMD was further decreased, but not significantly, by −0.32% at the femur neck and by −0.60% at the total hip in the lobeglitazone group, and these changes did not significantly differ compared with the original placebo group switched to lobeglitazone.
CONCLUSION
Our results indicate that treatment with lobeglitazone 0.5 mg over 52 weeks showed no detrimental effect on the BMD compared to the placebo.
MeSH Terms
Figure
Cited by 3 articles
-
Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes Metab J. 2021;45(3):326-336. doi: 10.4093/dmj.2020.0272.Recent Perspective on Thiazolidinedione
Won Jun Kim
J Korean Diabetes. 2021;22(2):97-104. doi: 10.4093/jkd.2021.22.2.97.A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Young Min, Sungrae Kim
Diabetes Metab J. 2022;46(6):855-865. doi: 10.4093/dmj.2021.0264.
Reference
-
1. Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Health ABC Study. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res. 2004; 19:1084–1091. PMID: 15176990.
Article2. Liao CC, Lin CS, Shih CC, Yeh CC, Chang YC, Lee YW, Chen TL. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care. 2014; 37:2246–2252. PMID: 24804698.
Article3. Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007; 22:1317–1328. PMID: 17501667.
Article4. Starup-Linde J, Frost M, Vestergaard P, Abrahamsen B. Epidemiology of fractures in diabetes. Calcif Tissue Int. 2017; 100:109–121. PMID: 27444009.
Article5. Choi YJ, Chung YS. Type 2 diabetes mellitus and bone fragility: special focus on bone imaging. Osteoporos Sarcopenia. 2016; 2:20–24.
Article6. Palermo A, D'Onofrio L, Eastell R, Schwartz AV, Pozzilli P, Napoli N. Oral anti-diabetic drugs and fracture risk, cut to the bone: safe or dangerous? A narrative review. Osteoporos Int. 2015; 26:2073–2089. PMID: 25910746.
Article8. Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012; 23:205–215. PMID: 22513163.
Article9. Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004; 145:401–406. PMID: 14500573.
Article10. Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010; 11:503–516. PMID: 20519122.11. Bilezikian JP, Josse RG, Eastell R, Lewiecki EM, Miller CG, Wooddell M, Northcutt AR, Kravitz BG, Paul G, Cobitz AR, Nino AJ, Fitzpatrick LA. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:1519–1528. PMID: 23450056.
Article12. Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014; 68:115–123. PMID: 25173606.
Article13. Billington EO, Grey A, Bolland MJ. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia. 2015; 58:2238–2246. PMID: 26109213.
Article14. Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, Tan MH, Khan MA, Perez AT, Jacober SJ. GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005; 28:1547–1554. PMID: 15983299.
Article15. Rabol R, Boushel R, Almdal T, Hansen CN, Ploug T, Haugaard SB, Prats C, Madsbad S, Dela F. Opposite effects of pioglitazone and rosiglitazone on mitochondrial respiration in skeletal muscle of patients with type 2 diabetes. Diabetes Obes Metab. 2010; 12:806–814. PMID: 20649633.16. Bone HG, Lindsay R, McClung MR, Perez AT, Raanan MG, Spanheimer RG. Effects of pioglitazone on bone in postmenopausal women with impaired fasting glucose or impaired glucose tolerance: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2013; 98:4691–4701. PMID: 24057294.
Article17. Li R, Xu W, Luo S, Xu H, Tong G, Zeng L, Zhu D, Weng J. Effect of exenatide, insulin and pioglitazone on bone metabolism in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 2015; 52:1083–1091. PMID: 26249206.
Article18. Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, Park JH, Park YS, Kim SJ, Choi DS. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014; 9:e92843. PMID: 24736628.
Article19. Kim SH, Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, Park JH, Park YS, Kim SJ, Choi DS. Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: an open-label extension study. Diabetes Res Clin Pract. 2015; 110:e27–e30. PMID: 26458774.
Article20. Fan B, Lu Y, Genant H, Fuerst T, Shepherd J. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int. 2010; 21:1227–1236. PMID: 19859644.
Article21. Kahn BB, McGraw TE. Rosiglitazone, PPARγ, and type 2 diabetes. N Engl J Med. 2010; 363:2667–2669. PMID: 21190462.
Article22. Home PD, Jones NP, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Komajda M, Curtis P. RECORD Study Group. Rosiglitazone RECORD study: glucose control outcomes at 18 months. Diabet Med. 2007; 24:626–634. PMID: 17517066.23. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O'Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR. IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016; 374:1321–1331. PMID: 26886418.
Article24. Liu J, Wang LN. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2015; (10):CD010693. PMID: 26511368.
Article25. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001; 19:180–192. PMID: 11359943.
Article26. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004; 3:379–389. PMID: 15569355.27. Patel JJ, Butters OR, Arnett TR. PPAR agonists stimulate adipogenesis at the expense of osteoblast differentiation while inhibiting osteoclast formation and activity. Cell Biochem Funct. 2014; 32:368–377. PMID: 24615887.
Article28. Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M. Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab. 2010; 12:716–721. PMID: 20590749.
Article29. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471. PMID: 17517853.
Article30. Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM. PROactive Investigators. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J Am Coll Cardiol. 2007; 49:1772–1780. PMID: 17466227.31. Osman I, Segar L. Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem Pharmacol. 2016; 101:54–70. PMID: 26643070.
Article32. Grey A, Bolland M, Fenwick S, Horne A, Gamble G, Drury PL, Reid IR. The skeletal effects of pioglitazone in type 2 diabetes or impaired glucose tolerance: a randomized controlled trial. Eur J Endocrinol. 2013; 170:255–262. PMID: 24217934.
Article33. Lee HW, Kim BY, Ahn JB, Kang SK, Lee JH, Shin JS, Ahn SK, Lee SJ, Yoon SS. Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur J Med Chem. 2005; 40:862–874. PMID: 15908051.
Article34. Kim BY, Ahn JB, Lee HW, Kang SK, Lee JH, Shin JS, Ahn SK, Hong CI, Yoon SS. Synthesis and biological activity of novel substituted pyridines and purines containing 2,4-thiazolidinedione. Eur J Med Chem. 2004; 39:433–447. PMID: 15110969.
Article35. Wu H, Li L, Ma Y, Chen Y, Zhao J, Lu Y, Shen P. Regulation of selective PPARγ modulators in the differentiation of osteoclasts. J Cell Biochem. 2013; 114:1969–1977. PMID: 23494891.
Article36. Okazaki R, Toriumi M, Fukumoto S, Miyamoto M, Fujita T, Tanaka K, Takeuchi Y. Thiazolidinediones inhibit osteoclastlike cell formation and bone resorption in vitro. Endocrinology. 1999; 140:5060–5065. PMID: 10537132.37. Weng J, Bi Y. Diabetes in China: the challenge now. J Diabetes Investig. 2010; 1:170–171.
Article38. Song SO, Lee YH, Kim DW, Song YD, Nam JY, Park KH, Kim DJ, Park SW, Lee HC, Lee BW. Trends in diabetes incidence in the last decade based on Korean National Health Insurance Claims Data. Endocrinol Metab (Seoul). 2016; 31:292–299. PMID: 27302715.
Article39. Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Lim S, Choi SH, Jang MJ, Kim Y, Oh K, Kim DJ, Cha BY. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J. 2013; 37:349–357. PMID: 24199164.
Article40. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011; 378:31–40. PMID: 21705069.
Article41. Bae KH, Seo JB, Jung YA, Seo HY, Kang SH, Jeon HJ, Lee JM, Lee S, Kim JG, Lee IK, Jung GS, Park KG. Lobeglitazone, a novel peroxisome proliferator-activated receptor γ agonist, attenuates renal fibrosis caused by unilateral ureteral obstruction in mice. Endocrinol Metab (Seoul). 2017; 32:115–123. PMID: 28256116.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
- Anti-Diabetic Medications and Osteoporosis
- Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
- Changes in the Bone Mineral Density of Femur Neck and Total Hip Over a 52-Week Treatment with Lobeglitazone
- Bone Mineral Density in Men with Type 2 Diabetes Mellitus