Korean J Orthod.
2016 Jul;46(4):228-241. 10.4041/kjod.2016.46.4.228.
Comparisons of orthodontic root resorption under heavy and jiggling reciprocating forces during experimental tooth movement in a rat model
- Affiliations
-
- 1Department of Orthodontics, Nihon University School of Dentistry at Matsudo, Japan. yamaguchi.masaru@nihon-u.ac.jp
- KMID: 2392203
- DOI: http://doi.org/10.4041/kjod.2016.46.4.228
Abstract
OBJECTIVE
Root mobility due to reciprocating movement of the tooth (jiggling) may exacerbate orthodontic root resorption (ORR). "Jiggling" describes mesiodistal or buccolingual movement of the roots of the teeth during orthodontic treatment. In the present study, buccolingual movement is described as "jiggling." We aimed to investigate the relationship between ORR and jiggling and to test for positive cell expression in odontoclasts in resorbed roots during experimental tooth movement (jiggling) in vivo.
METHODS
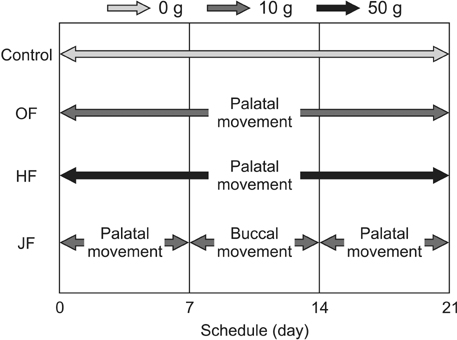
Male Wistar rats were divided into control, heavy force (HF), optimal force (OF), and jiggling force (JF) groups. The expression levels of cathepsin K, matrix metalloproteinase (MMP)-9 protein, interleukin (IL)-6, cytokine-induced neutrophil chemoattractant 1 (CINC-1; an IL-8-related protein in rodents), receptor activator of nuclear factor κB ligand (RANKL), and osteoprotegerin protein in the dental root were determined using immunohistochemistry.
RESULTS
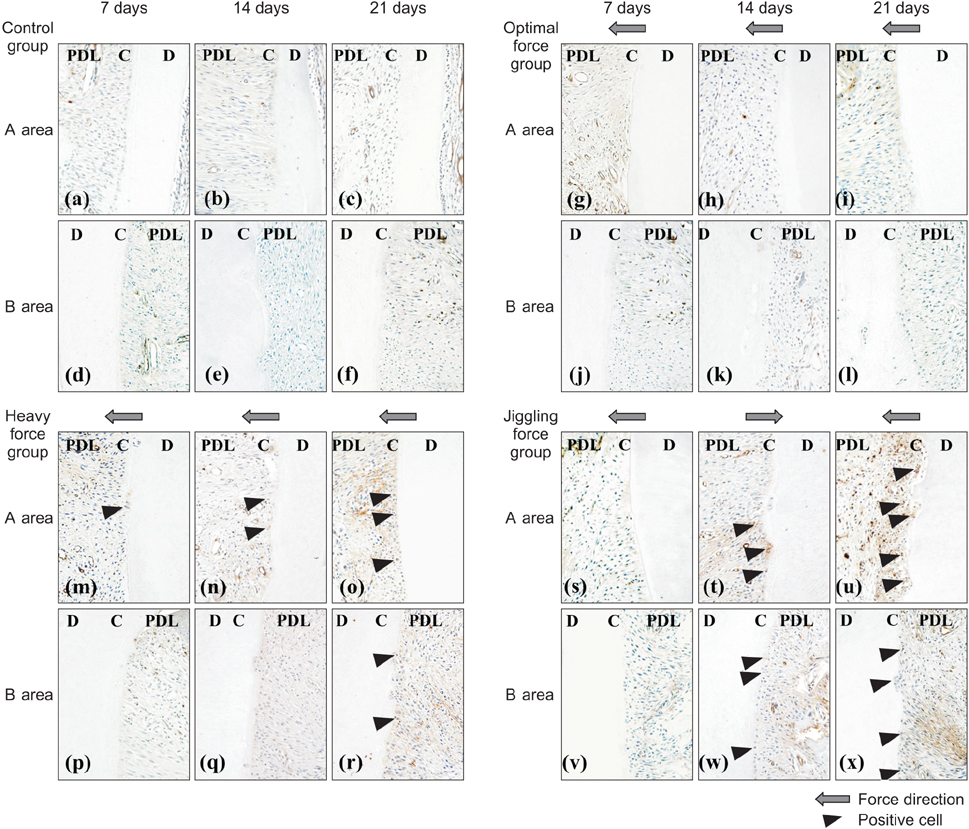
On day 21, a greater number of root resorption lacunae, which contained multinucleated odontoclasts, were observed in the palatal roots of rats in the JF group than in rats from other groups. Furthermore, there was a significant increase in the numbers of cathepsin K-positive and MMP-9-positive odontoclasts in the JF group on day 21. Immunoreactivities for IL-6, CINC-1, and RANKL were stronger in resorbed roots exposed to jiggling than in the other groups on day 21. Negative reactivity was observed in the controls.
CONCLUSIONS
These results suggest that jiggling may induce ORR via inflammatory cytokine production during orthodontic tooth movement, and that jiggling may be a risk factor for ORR.
MeSH Terms
Figure
Cited by 2 articles
-
Cone-beam computed tomography for the assessment of root–crown ratios of the maxillary and mandibular incisors in a Korean population
Sung-Hwan Choi, Jung-Suk Kim, Cheol-Soon Kim, Hyung-Seog Yu, Chung-Ju Hwang
Korean J Orthod. 2017;47(1):39-49. doi: 10.4041/kjod.2017.47.1.39.Effect of caspases and RANKL induced by heavy force in orthodontic root resorption
Yukari Minato, Masaru Yamaguchi, Mami Shimizu, Jun Kikuta, Takuji Hikida, Momoko Hikida, Masaaki Suemitsu, Kayo Kuyama, Kazutaka Kasai
Korean J Orthod. 2018;48(4):253-261. doi: 10.4041/kjod.2018.48.4.253.
Reference
-
1. Kaley J, Phillips C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991; 61:125–132.2. Malmgren O, Goldson L, Hill C, Orwin A, Petrini L, Lundberg M. Root resorption after orthodontic treatment of traumatized teeth. Am J Orthod. 1982; 82:487–491.
Article3. Linge BO, Linge L. Apical root resorption in upper anterior teeth. Eur J Orthod. 1983; 5:173–183.
Article4. Owman-Moll P, Kurol J, Lundgren D. The effects of a four-fold increased orthodontic force magnitude on tooth movement and root resorptions. An intraindividual study in adolescents. Eur J Orthod. 1996; 18:287–294.
Article5. Owman-Moll P, Kurol J, Lundgren D. Effects of a doubled orthodontic force magnitude on tooth movement and root resorptions. An inter-individual study in adolescents. Eur J Orthod. 1996; 18:141–150.
Article6. Chan E, Darendeliler MA. Physical properties of root cementum: Part 5. Volumetric analysis of root resorption craters after application of light and heavy orthodontic forces. Am J Orthod Dentofacial Orthop. 2005; 127:186–195.
Article7. Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 2. Literature review. Am J Orthod Dentofacial Orthop. 1993; 103:138–146.
Article8. Mirabella AD, Artun J. Risk factors for apical root resorption of maxillary anterior teeth in adult orthodontic patients. Am J Orthod Dentofacial Orthop. 1995; 108:48–55.
Article9. Hayashi A, Hayashi H, Kawata T. Prevention of root resorption in hypofunctional teeth by occlusal function recovery. Angle Orthod. 2015; DOI: 10.2319/012215-47.1. [Epub ahead of print].
Article10. Nakano Y, Yamaguchi M, Fujita S, Asano M, Saito K, Kasai K. Expressions of RANKL/RANK and M-CSF/c-fms in root resorption lacunae in rat molar by heavy orthodontic force. Eur J Orthod. 2011; 33:335–343.
Article11. Kaku M, Sumi H, Shikata H, Kojima S, Motokawa M, Fujita T, et al. Effects of pulpectomy on the amount of root resorption during orthodontic tooth movement. J Endod. 2014; 40:372–378.
Article12. Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008; 78:502–509.
Article13. Kohno T, Matsumoto Y, Kanno Z, Warita H, Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J Orthod. 2002; 29:129–135.
Article14. Gameiro GH, Nouer DF, Pereira-Neto JS, de Araújo Magnani MB, de Andrade ED, Novaes PD, et al. Histological analysis of orthodontic root resorption in rats treated with the cyclooxygenase-2 (COX-2) inhibitor celecoxib. Orthod Craniofac Res. 2008; 11:156–161.
Article15. Eross E, Turk T, Elekdag-Turk S, Cakmak F, Jones AS, Végh A, et al. Physical properties of root cementum: Part 25. Extent of root resorption after the application of light and heavy buccopalatal jiggling forces for 12 weeks: A microcomputed tomography study. Am J Orthod Dentofacial Orthop. 2015; 147:738–746.
Article16. Brudvik P, Rygh P. Non-clast cells start orthodontic root resorption in the periphery of hyalinized zones. Eur J Orthod. 1993; 15:467–480.
Article17. Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 1. Literature review. Am J Orthod Dentofacial Orthop. 1993; 103:62–66.
Article18. Brudvik P, Rygh P. Root resorption beneath the main hyalinized zone. Eur J Orthod. 1994; 16:249–263.
Article19. Kurol J, Owman-Moll P. Hyalinization and root resorption during early orthodontic tooth movement in adolescents. Angle Orthod. 1998; 68:161–165.20. Kvam E. Scanning electron microscopy of tissue changes on the pressure surface of human premolars following tooth movement. Scand J Dent Res. 1972; 80:357–368.
Article21. Rygh P. Ultrastructural cellular reactions in pressure zones of rat molar periodontium incident to orthodontic tooth movement. Acta Odontol Scand. 1972; 30:575–593.
Article22. Ohashi M, Yamaguchi M, Hikida T, Kikuta J, Shimizu M, Goseki T, et al. Jiggling force induces orthodontic root resorption during tooth movement in rats. Int Oral-Med Sci. 2015; 14:13–20.
Article23. Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 2006; 28:221–240.
Article24. Wucherpfennig AL, Li YP, Stetler-Stevenson WG, Rosenberg AE, Stashenko P. Expression of 92 kD type IV collagenase/gelatinase B in human osteoclasts. J Bone Miner Res. 1994; 9:549–556.
Article25. Oshiro T, Shibasaki Y, Martin TJ, Sasaki T. Immunolocalization of vacuolar-type H+-ATPase, cathepsin K, matrix metalloproteinase-9, and receptor activator of NFkappaB ligand in odontoclasts during physiological root resorption of human deciduous teeth. Anat Rec. 2001; 264:305–311.
Article26. Tsuchiya M, Akiba Y, Takahashi I, Sasano Y, Kashiwazaki J, Tsuchiya S, et al. Comparison of expression patterns of cathepsin K and MMP-9 in odontoclasts and osteoclasts in physiological root resorption in the rat molar. Arch Histol Cytol. 2008; 71:89–100.
Article27. Asano M, Yamaguchi M, Nakajima R, Fujita S, Utsunomiya T, Yamamoto H, et al. IL-8 and MCP-1 induced by excessive orthodontic force mediates odontoclastogenesis in periodontal tissues. Oral Dis. 2011; 17:489–498.
Article28. Hayashi N, Yamaguchi M, Nakajima R, Utsunomiya T, Yamamoto H, Kasai K. T-helper 17 cells mediate the osteo/odontoclastogenesis induced by excessive orthodontic forces. Oral Dis. 2012; 18:375–388.
Article29. Kikuta J, Yamaguchi M, Shimizu M, Yoshino T, Kasai K. Notch signaling induces root resorption via RANKL and IL-6 from hPDL cells. J Dent Res. 2015; 94:140–147.
Article30. Garlet TP, Coelho U, Repeke CE, Silva JS, Cunha Fde Q, Garlet GP. Differential expression of osteoblast and osteoclast chemmoatractants in compression and tension sides during orthodontic movement. Cytokine. 2008; 42:330–335.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in the titer of tooth root antibodies accompanying root resorption associated with orthodontic tooth movement
- Root resorption and bone resorption by jiggling force in cat premolars
- A study of root resorption and alveolar bone changes during tooth movement after treatment with etidronate disodium
- Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption
- A study on the affecting factors on root resorption