J Clin Neurol.
2017 Oct;13(4):387-393. 10.3988/jcn.2017.13.4.387.
Functional Connectivity of the Hippocampus in Early- and vs. Late-Onset Alzheimer's Disease
- Affiliations
-
- 1Department of Neurology, Gachon University Gil Medical Center, Incheon, Korea. khpark@gachon.ac.kr
- 2Neuroscience Research Institute, Gachon University, Incheon, Korea. ydson@gachon.ac.kr
- 3Department of Family Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 4Department of Radiology, Gachon University Gil Medical Center, Incheon, Korea.
- 5Department of Biomedical Engineering, College of Health Science, Gachon University, Incheon, Korea.
- KMID: 2392135
- DOI: http://doi.org/10.3988/jcn.2017.13.4.387
Abstract
- BACKGROUND AND PURPOSE
Early-onset Alzheimer's disease (EOAD) and late-onset Alzheimer's disease (LOAD) have different clinical and neuroimaging characteristics, but memory decline is usually present in both types. However, there have been few functional studies focused on the hippocampus in Alzheimer's disease. We therefore investigated the functional connectivity between the hippocampus and other brain regions using resting-state fMRI and compared the findings between EOAD and LOAD.
METHODS
We recruited 13 patients with EOAD and 19 patients with LOAD at the early disease stage. Twenty-one young controls and ten old controls were also recruited. Each participant completed a standardized neuropsychological battery of tests and underwent T1-weighted structural MRI. fMRI data were acquired during the resting state using 3-T MRI. The functional connectivity to the hippocampus was calculated based on automated anatomical labeling templates.
RESULTS
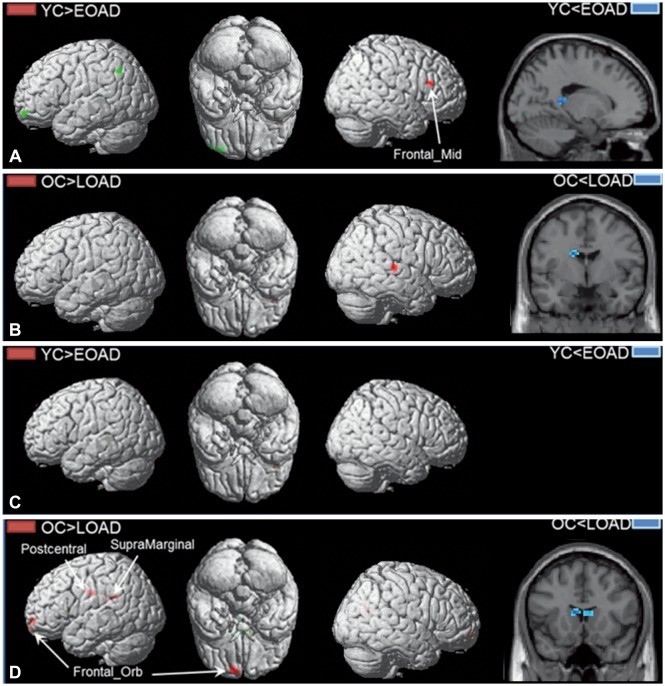
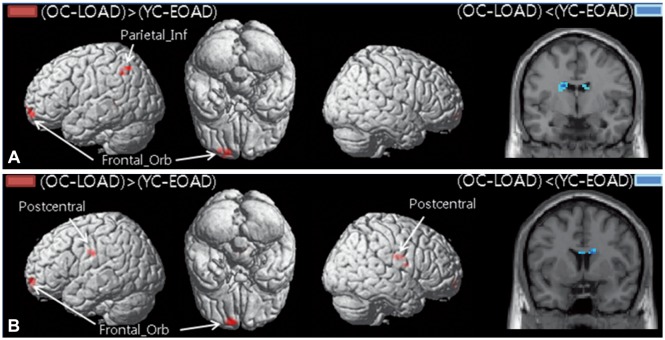
The functional connectivity from the hippocampus to other brain regions differed between patients with EOAD and LOAD. The LOAD patients showed decreased hippocampal connectivity to cortical regions, such as to the middle temporal cortex, orbitofrontal cortex, postcentral cortex, supramarginal cortex, and rolandic operculum. In contrast, EOAD patients showed smaller functional changes of the cortical regions connected to the hippocampus, such as the middle frontal cortex.
CONCLUSIONS
EOAD and LOAD patients exhibited different hippocampal connectivity. The memory decline in EOAD may be due to brain areas other than the hippocampus.
Keyword
MeSH Terms
Figure
Reference
-
1. Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993; 33:403–408. PMID: 8307060.
Article2. Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009; 72:999–1007. PMID: 19289740.
Article3. Barnes J, Bartlett JW, van de, Loy CT, Scahill RI, Frost C, et al. A meta-analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging. 2009; 30:1711–1723. PMID: 18346820.
Article4. Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006; 27:1372–1384. PMID: 16289476.
Article5. Fellgiebel A, Yakushev I. Diffusion tensor imaging of the hippocampus in MCI and early Alzheimer's disease. J Alzheimers Dis. 2011; 26(Suppl 3):257–262. PMID: 21971465.
Article6. Hong YJ, Yoon B, Lim SC, Shim YS, Kim JY, Ahn KJ, et al. Microstructural changes in the hippocampus and posterior cingulate in mild cognitive impairment and Alzheimer's disease: a diffusion tensor imaging study. Neurol Sci. 2013; 34:1215–1221. PMID: 23109096.
Article7. Rombouts S, Scheltens P. Functional connectivity in elderly controls and AD patients using resting state fMRI: a pilot study. Curr Alzheimer Res. 2005; 2:115–116. PMID: 15974906.
Article8. Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, et al. Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum Brain Mapp. 2007; 28:967–978. PMID: 17133390.
Article9. Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006; 31:496–504. PMID: 16473024.
Article10. van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer's disease: the case of the missing APOE ε4 allele. Lancet Neurol. 2011; 10:280–288. PMID: 21185234.
Article11. Cho H, Jeon S, Kang SJ, Lee JM, Lee JH, Kim GH, et al. Longitudinal changes of cortical thickness in early-versus late-onset Alzheimer's disease. Neurobiol Aging. 2013; 34:1921.e9–1921.e15.12. Ridgway GR, Lehmann M, Barnes J, Rohrer JD, Warren JD, Crutch SJ, et al. Early-onset Alzheimer disease clinical variants: multivariate analyses of cortical thickness. Neurology. 2012; 79:80–84. PMID: 22722624.
Article13. Cho H, Seo SW, Kim JH, Kim C, Ye BS, Kim GH, et al. Changes in subcortical structures in early-versus late-onset Alzheimer's disease. Neurobiol Aging. 2013; 34:1740–1747. PMID: 23394958.14. Moon SW, Dinov ID, Hobel S, Zamanyan A, Choi YC, Shi R, et al. Structural brain changes in early-onset Alzheimer's disease subjects using the LONI pipeline environment. J Neuroimaging. 2015; 25:728–737. PMID: 25940587.
Article15. Kim EJ, Cho SS, Jeong Y, Park KC, Kang SJ, Kang E, et al. Glucose metabolism in early onset versus late onset Alzheimer's disease: an SPM analysis of 120 patients. Brain. 2005; 128:1790–1801. PMID: 15888536.
Article16. Kaiser NC, Melrose RJ, Liu C, Sultzer DL, Jimenez E, Su M, et al. Neuropsychological and neuroimaging markers in early vs. late-onset Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2012; 27:520–529. PMID: 22990206.17. Cho H, Seo SW, Kim JH, Suh MK, Lee JH, Choe YS, et al. Amyloid deposition in early onset versus late onset Alzheimer's disease. J Alzheimers Dis. 2013; 35:813–821. PMID: 23507771.
Article18. Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011; 10:785–796. PMID: 21802369.
Article19. Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, et al. Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology. 2014; 83:1936–1944. PMID: 25344382.
Article20. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurology. 1984; 34:939–944. PMID: 6610841.
Article21. Noh Y, Seo SW, Jeon S, Lee JM, Kim JH, Kim GH, et al. White matter hyperintensities are associated with amyloid burden in APOE4 non-carriers. J Alzheimers Dis. 2014; 40:877–886. PMID: 24577457.
Article22. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010; 25:1071–1076. PMID: 20592901.
Article23. Sohn WS, Yoo K, Na DL, Jeong Y. Progressive changes in hippocampal resting-state connectivity across cognitive impairment: a cross-sectional study from normal to Alzheimer disease. Alzheimer Dis Assoc Disord. 2014; 28:239–246. PMID: 24614267.24. Frisoni GB, Pievani M, Testa C, Sabattoli F, Bresciani L, Bonetti M, et al. The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain. 2007; 130:720–730. PMID: 17293358.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Three Large-Scale Functional Brain Networks from Resting-State Functional MRI in Subjects with Different Levels of Cognitive Impairment
- Brain MRI Findings for the Patient with the Late Onset Schizophrenia: Comparison among Patients with the Early Onset Schizophrenia, Progressive Schizophrenia, Senile Dementia and Controls
- Apolipoprotein E4: A Risk Factor for Successful Cognitive Aging
- Changes in the Hippocampal Volume and Shape in Early-Onset Mild Cognitive Impairment
- Pathogenic Mechanism of Alzheimer's Disease