Clin Exp Vaccine Res.
2013 Jan;2(1):8-18.
Requirements for improved vaccines against foot-and-mouth disease epidemics
- Affiliations
-
- 1Animal, Plant, and Fisheries Quarantine and Inspection Agency, Anyang, Korea. parkjhvet@korea.kr
Abstract
- Inactivated foot-and-mouth disease (FMD) vaccines are currently used worldwide. With the emergence of various FMD virus serotypes and subtypes, vaccines must become more suitable for field-based uses under the current circumstances in terms of the fast and proper selection of vaccine strains, an extended vaccine development period for new viruses, protecting against the risk of virus leakage during vaccine manufacture, counteracting the delayed onset of immune response, counteracting shorter durations of immunity, and the accurate serological differentiation of infected and vaccinated animals and multiple vaccination. The quality of vaccines should then be improved to effectively control FMD outbreaks and minimize the problems that can arise among livestock after vaccinations. Vaccine improvement should be based on using attenuated virus strains with high levels of safety. Moreover, when vaccines are urgently required for newly spread field strains, the seed viruses for new vaccines should be developed for only a short period. Improved vaccines should offer superior immunization to all susceptible animals including cattle and swine. In addition, they should have highly protective effects without persistent infection. In this way, if vaccines are developed using new methods such as reverse genetics or vector vaccine technology, in which live viruses can be easily made by replacing specific protective antigens, even a single vaccination is likely to generate highly protective effects with an extended duration of immunity, and the safety and stability of the vaccines will be assured. We therefore reviewed the current FMD vaccines and their adjuvants, and evaluated if they provide superior immunization to all susceptible animals including cattle and swine.
Keyword
MeSH Terms
Figure
Reference
-
1. Bachrach HL. Foot-and-mouth disease. Annu Rev Microbiol. 1968. 22:201–244.
Article2. Hedger RS, Taylor WP, Barnett IT, Riek R, Harpham D. Simultaneous vaccination of cattle against foot-and-mouth disease and rinderpest. Trop Anim Health Prod. 1986. 18:21–25.
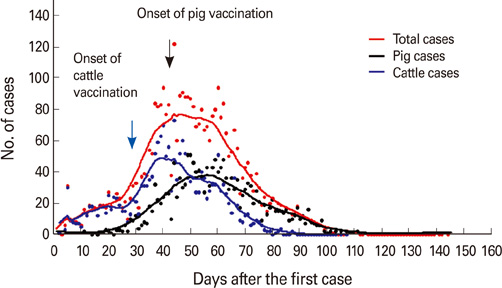
Article3. Yoon H, Yoon SS, Wee SH, Kim YJ, Kim B. Clinical manifestations of foot-and-mouth disease during the 2010/2011 epidemic in the Republic of Korea. Transbound Emerg Dis. 2012. 59:517–525.
Article4. Dupuis L, Ascarateil S, Aucouturier J, Ganne V. SEPPIC vaccine adjuvants for poultry. Ann N Y Acad Sci. 2006. 1081:202–205.
Article5. Cloete M, Dungu B, Van Staden LI, Ismail-Cassim N, Vosloo W. Evaluation of different adjuvants for foot-and-mouth disease vaccine containing all the SAT serotypes. Onderstepoort J Vet Res. 2008. 75:17–31.
Article6. McKercher PD, Graves JH. A review of the current status of oil adjuvants in foot-and-mouth disease vaccines. Dev Biol Stand. 1976. 35:107–112.7. Andersen AA, Campbell CH. Experimental placental transfer of foot-and-mouth disease virus in mice. Am J Vet Res. 1976. 37:585–589.8. Mackenzie JS, Slade WR. Evidence for recombination between two different immunological types of foot-and-mouth disease virus. Aust J Exp Biol Med Sci. 1975. 53:251–256.
Article9. Knowles NJ, He J, Shang Y, et al. Southeast Asian foot-and-mouth disease viruses in Eastern Asia. Emerg Infect Dis. 2012. 18:499–501.
Article10. Parida S. Vaccination against foot-and-mouth disease virus: strategies and effectiveness. Expert Rev Vaccines. 2009. 8:347–365.
Article11. Madhanmohan M, Nagendrakumar SB, Kumar R, et al. Clinical protection, sub-clinical infection and persistence following vaccination with extinction payloads of O1 Manisa foot-and-mouth disease monovalent vaccine and challenge in goats and comparison with sheep. Res Vet Sci. 2012. 93:1050–1059.
Article12. Doel TR. Optimisation of the immune response to foot-and-mouth disease vaccines. Vaccine. 1999. 17:1767–1771.
Article13. Doel TR. FMD vaccines. Virus Res. 2003. 91:81–99.
Article14. Mowat GN, Barr DA, Bennett JH. The development of an attenuated foot-and-mouth disease virus vaccine by modification and cloning in tissue cultures of BHK21 cells. Arch Gesamte Virusforsch. 1969. 26:341–354.
Article15. Polacino P, Kaplan G, Yafal AG, Palma EL. Biochemical characterization of a foot-and-mouth disease virus strain attenuated for cattle: brief report. Arch Virol. 1986. 88:143–150.
Article16. Saiz M, Nunez JI, Jimenez-Clavero MA, Baranowski E, Sobrino F. Foot-and-mouth disease virus: biology and prospects for disease control. Microbes Infect. 2002. 4:1183–1192.
Article17. Doel TR. Natural and vaccine-induced immunity to foot and mouth disease: the prospects for improved vaccines. Rev Sci Tech. 1996. 15:883–911.
Article18. Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001. 19:2666–2672.
Article19. Barnett PV, Pullen L, Williams L, Doel TR. International bank for foot-and-mouth disease vaccine: assessment of Montanide ISA 25 and ISA 206, two commercially available oil adjuvants. Vaccine. 1996. 14:1187–1198.
Article20. Yeruham I, Yadin H, Haymovich M, Perl S. Adverse reactions to FMD vaccine. Vet Dermatol. 2001. 12:197–201.
Article21. McCullough KC, Sobrino F. Sobrino F, Domingo E, editors. Immunology of foot-and-mouth disease. Foot and mouth disease: current perspectives. 2004. Norfolk: Horizon Biosciences;173–222.
Article22. Spath EJ, Smitsaart E, Casaro AP, et al. Immune response of calves to foot-and-mouth disease virus vaccine emulsified with oil adjuvant: strategies of vaccination. Vaccine. 1995. 13:909–914.
Article23. Mason PW, Piccone ME, McKenna TS, Chinsangaram J, Grubman MJ. Evaluation of a live-attenuated foot-and-mouth disease virus as a vaccine candidate. Virology. 1997. 227:96–102.
Article24. Chinsangaram J, Mason PW, Grubman MJ. Protection of swine by live and inactivated vaccines prepared from a leader proteinase-deficient serotype A12 foot-and-mouth disease virus. Vaccine. 1998. 16:1516–1522.
Article25. Jamal SM, Ferrari G, Ahmed S, Normann P, Belsham GJ. Molecular characterization of serotype Asia-1 foot-and-mouth disease viruses in Pakistan and Afghanistan: emergence of a new genetic group and evidence for a novel recombinant virus. Infect Genet Evol. 2011. 11:2049–2062.
Article26. Lee KN, Oem JK, Park JH, et al. Evidence of recombination in a new isolate of foot-and-mouth disease virus serotype Asia 1. Virus Res. 2009. 139:117–121.
Article27. Lewis-Rogers N, McClellan DA, Crandall KA. The evolution of foot-and-mouth disease virus: impacts of recombination and selection. Infect Genet Evol. 2008. 8:786–798.
Article28. Rodriguez LL, Gay CG. Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert Rev Vaccines. 2011. 10:377–387.
Article29. Li P, Bai X, Sun P, et al. Evaluation of a genetically modified foot-and-mouth disease virus vaccine candidate generated by reverse genetics. BMC Vet Res. 2012. 8:57.
Article30. Li P, Bai X, Lu Z, et al. Construction of a full-length infectious cDNA clone of inter-genotypic chimeric foot-and-mouth disease virus. Wei Sheng Wu Xue Bao. 2012. 52:114–119.31. Pacheco JM, Piccone ME, Rieder E, Pauszek SJ, Borca MV, Rodriguez LL. Domain disruptions of individual 3B proteins of foot-and-mouth disease virus do not alter growth in cell culture or virulence in cattle. Virology. 2010. 405:149–156.
Article32. Uddowla S, Hollister J, Pacheco JM, Rodriguez LL, Rieder E. A safe foot-and-mouth disease vaccine platform with two negative markers for differentiating infected from vaccinated animals. J Virol. 2012. 86:11675–11685.
Article33. Li S, Gao M, Zhang R, et al. A mutant of infectious Asia 1 serotype foot-and-mouth disease virus with the deletion of 10-amino-acid in the 3A protein. Virus Genes. 2010. 41:406–413.
Article34. Piccone ME, Pacheco JM, Pauszek SJ, et al. The region between the two polyprotein initiation codons of foot-and-mouth disease virus is critical for virulence in cattle. Virology. 2010. 396:152–159.
Article35. Li P, Bai X, Cao Y, et al. Expression and stability of foreign epitopes introduced into 3A nonstructural protein of foot-and-mouth disease virus. PLoS One. 2012. 7:e41486.
Article36. de Avila Botton S, Brum MC, Bautista E, et al. Immunopotentiation of a foot-and-mouth disease virus subunit vaccine by interferon alpha. Vaccine. 2006. 24:3446–3456.
Article37. Moraes MP, Chinsangaram J, Brum MC, Grubman MJ. Immediate protection of swine from foot-and-mouth disease: a combination of adenoviruses expressing interferon alpha and a foot-and-mouth disease virus subunit vaccine. Vaccine. 2003. 22:268–279.
Article38. Martin-Acebes MA, Vazquez-Calvo A, Rincon V, Mateu MG, Sobrino F. A single amino acid substitution in the capsid of foot-and-mouth disease virus can increase acid resistance. J Virol. 2011. 85:2733–2740.
Article39. Hegde NR, Maddur MS, Rao PP, Kaveri SV, Bayry J. Thermostable foot-and-mouth disease virus as a vaccine candidate for endemic countries: a perspective. Vaccine. 2009. 27:2199–2201.
Article40. Zhu J, Weiss M, Grubman MJ, de los Santos T. Differential gene expression in bovine cells infected with wild type and leaderless foot-and-mouth disease virus. Virology. 2010. 404:32–40.
Article41. Almeida MR, Rieder E, Chinsangaram J, et al. Construction and evaluation of an attenuated vaccine for foot-and-mouth disease: difficulty adapting the leader proteinase-deleted strategy to the serotype O1 virus. Virus Res. 1998. 55:49–60.
Article42. Brown CC, Piccone ME, Mason PW, McKenna TS, Grubman MJ. Pathogenesis of wild-type and leaderless foot-and-mouth disease virus in cattle. J Virol. 1996. 70:5638–5641.
Article43. Cottam EM, Wadsworth J, Shaw AE, et al. Transmission pathways of foot-and-mouth disease virus in the United Kingdom in 2007. PLoS Pathog. 2008. 4:e1000050.
Article44. Brake DA, McIlhaney M, Miller T, et al. Human adenovirus-vectored foot-and-mouth disease vaccines: establishment of a vaccine product profile through in vitro testing. Dev Biol (Basel). 2012. 134:123–133.45. D'Antuono A, Laimbacher AS, La Torre J, et al. HSV-1 amplicon vectors that direct the in situ production of foot-and-mouth disease virus antigens in mammalian cells can be used for genetic immunization. Vaccine. 2010. 28:7363–7372.46. Ren XG, Xue F, Zhu YM, et al. Construction of a recombinant BHV-1 expressing the VP1 gene of foot and mouth disease virus and its immunogenicity in a rabbit model. Biotechnol Lett. 2009. 31:1159–1165.
Article47. Wu Q, Moraes MP, Grubman MJ. Recombinant adenovirus co-expressing capsid proteins of two serotypes of foot-and-mouth disease virus (FMDV): in vitro characterization and induction of neutralizing antibodies against FMDV in swine. Virus Res. 2003. 93:211–219.
Article48. Grubman MJ, Mason PW. Prospects, including time-frames, for improved foot and mouth disease vaccines. Rev Sci Tech. 2002. 21:589–600.
Article49. Mohana Subramanian B, Madhanmohan M, Sriraman R, et al. Development of foot-and-mouth disease virus (FMDV) serotype O virus-like-particles (VLPs) vaccine and evaluation of its potency. Antiviral Res. 2012. 96:288–295.
Article50. Remond M, Da Costa B, Riffault S, et al. Infectious bursal disease subviral particles displaying the foot-and-mouth disease virus major antigenic site. Vaccine. 2009. 27:93–98.
Article51. Capozzo AV, Wilda M, Bucafusco D, et al. Vesicular stomatitis virus glycoprotein G carrying a tandem dimer of foot and mouth disease virus antigenic site A can be used as DNA and peptide vaccine for cattle. Antiviral Res. 2011. 92:219–227.
Article52. Li Z, Yi Y, Yin X, Zhang Z, Liu J. Expression of foot-and-mouth disease virus capsid proteins in silkworm-baculovirus expression system and its utilization as a subunit vaccine. PLoS One. 2008. 3:e2273.
Article53. Li G, Chen W, Yan W, et al. Comparison of immune responses against foot-and-mouth disease virus induced by fusion proteins using the swine IgG heavy chain constant region or beta-galactosidase as a carrier of immunogenic epitopes. Virology. 2004. 328:274–281.
Article54. Mateo R, Luna E, Rincon V, Mateu MG. Engineering viable foot-and-mouth disease viruses with increased thermostability as a step in the development of improved vaccines. J Virol. 2008. 82:12232–12240.
Article55. Li D. Chitosan can stop or postpone the death of the suckling mice challenged with foot-and-mouth disease virus. Virol J. 2010. 7:125.
Article56. Quattrocchi V, Bianco V, Fondevila N, Pappalardo S, Sadir A, Zamorano P. Use of new adjuvants in an emergency vaccine against foot-and-mouth disease virus: evaluation of conferred immunity. Dev Biol (Basel). 2004. 119:481–497.57. Batista A, Quattrocchi V, Olivera V, et al. Adjuvant effect of Cliptox on the protective immune response induced by an inactivated vaccine against foot and mouth disease virus in mice. Vaccine. 2010. 28:6361–6366.
Article58. Jaworski JP, Compaired D, Trotta M, Perez M, Trono K, Fondevila N. Validation of an r3AB1-FMDV-NSP ELISA to distinguish between cattle infected and vaccinated with foot-and-mouth disease virus. J Virol Methods. 2011. 178:191–200.
Article59. Office International des Epizooties. Report of the Meeting of the OIE Ad Hoc Group on Evaluation of Nonstructural Protein Tests for Foot and Mouth Disease Diagnosis. 2002. 2002 Oct 2-4; Paris. Paris: Office International des Epizooties.60. OIE World Organisation for Animal Health. Terrestrial Animal Health Code. Chapter 8.5. Foot and mouth disease. 2012. Paris: OIE World Organisation for Animal Health.61. Sorensen KJ, Madsen KG, Madsen ES, Salt JS, Nqindi J, Mackay DK. Differentiation of infection from vaccination in foot-and-mouth disease by the detection of antibodies to the non-structural proteins 3D, 3AB and 3ABC in ELISA using antigens expressed in baculovirus. Arch Virol. 1998. 143:1461–1476.
Article62. McCullough KC, De Simone F, Brocchi E, Capucci L, Crowther JR, Kihm U. Protective immune response against foot-and-mouth disease. J Virol. 1992. 66:1835–1840.
Article63. Black L, Francis MJ, Rweyemamu MM, Umebara O, Boge A. The relationship between serum antibody titres and protection from foot and mouth disease in pigs after oil emulsion vaccination. J Biol Stand. 1984. 12:379–389.
Article64. OIE World Organisation for Animal Health. Manual of diagnostic tests and vaccines for terrestrial animals. 2012. Paris: OIE World Organisation for Animal Health.65. Parida S, Oh Y, Reid SM, et al. Interferon-gamma production in vitro from whole blood of foot-and-mouth disease virus (FMDV) vaccinated and infected cattle after incubation with inactivated FMDV. Vaccine. 2006. 24:964–969.
Article66. Sanz-Parra A, Jimenez-Clavero MA, Garcia-Briones MM, Blanco E, Sobrino F, Ley V. Recombinant viruses expressing the foot-and-mouth disease virus capsid precursor polypeptide (P1) induce cellular but not humoral antiviral immunity and partial protection in pigs. Virology. 1999. 259:129–134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influenza
- Effect of simultaneous administration of foot-and-mouth disease (FMD) and anthrax vaccines on antibody response to FMD in sheep
- Novel foot-and-mouth disease virus in Korea, July-August 2014
- QS-21 enhances the early antibody response to oil adjuvant foot-and-mouth disease vaccine in cattle
- Enterovirus 71 infection and vaccines