Nutr Res Pract.
2013 Apr;7(2):89-95.
Inorganic sulfur reduces cell proliferation by inhibiting of ErbB2 and ErbB3 protein and mRNA expression in MDA-MB-231 human breast cancer cells
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, 126 Jukjeon-dong, Suji-gu, Yongin-si, Gyunggi 448-701, Korea. wkkim@dankook.ac.kr
- 2Department of Food and Nutrition, Beawha Women's University, Seoul 110-735, Korea.
Abstract
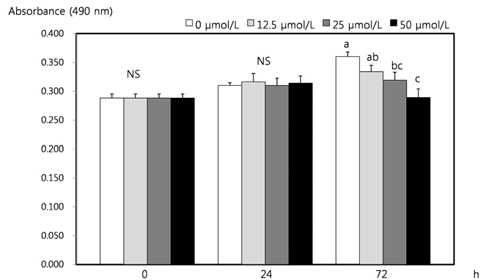
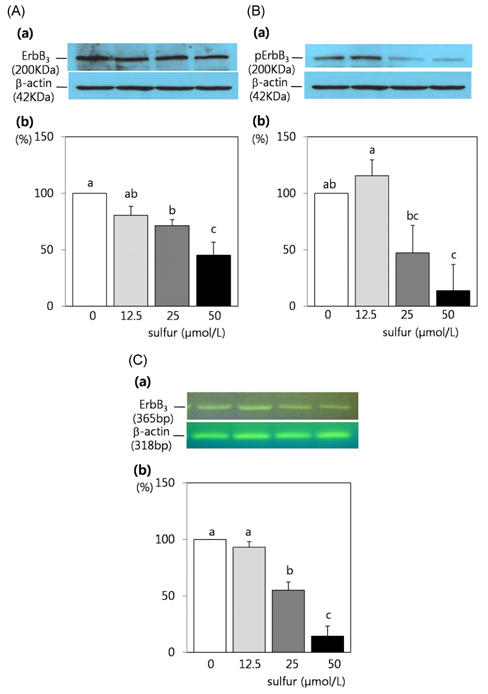
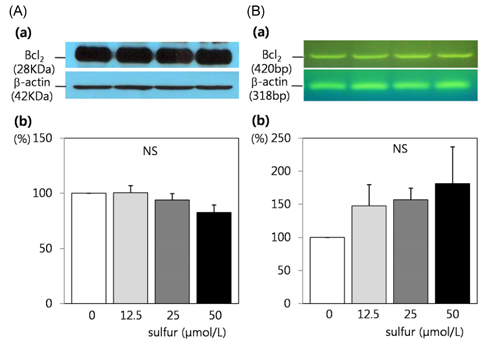
- Dietary inorganic sulfur is the minor component in our diet, but some studies suggested that inorganic sulfur is maybe effective to treat cancer related illness. Therefore, this study aims to examine the effects of inorganic sulfur on cell proliferation and gene expression in MDA-MB-231 human breast cancer cells. MDA-MB-231 cells were cultured the absence or presence of various concentrations (12.5, 25, or 50 micromol/L) of inorganic sulfur. Inorganic sulfur significantly decreased proliferation after 72 h of incubation (P < 0.05). The protein expression of ErbB2 and its active form, pErbB2, were significantly reduced at inorganic sulfur concentrations of 50 micromol/L and greater than 25 micromol/L, respectively (P < 0.05). The mRNA expression of ErbB2 was significantly reduced at an inorganic sulfur concentration of 50 micromol/L (P < 0.05). The protein expression of ErbB3 and its active form, pErbB3, and the mRNA expression of ErbB3 were significantly reduced at inorganic sulfur concentrations greater than 25 micromol/L (P < 0.05). The protein and mRNA expression of Akt were significantly reduced at an inorganic sulfur concentration of 50 micromol/L (P < 0.05), but pAkt was not affected by inorganic sulfur treatment. The protein and mRNA expression of Bax were significantly increased with the addition of inorganic sulfur concentration of 50 micromol/L (P < 0.05). In conclusion, cell proliferation was suppressed by inorganic sulfur treatment through the ErbB-Akt pathway in MDA-MB-231 cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Ingenbleek Y. The nutritional relationship linking sulfur to nitrogen in living organisms. J Nutr. 2006. 136:1641S–1651S.
Article2. Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000. 72:1488–1494.
Article3. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988. 18:553–558.
Article4. Sulfur. National Institute of Food and Drug Safety Evaluation [Internet]. 2010. cited 2010 August 10. Cheongwon: National Institute of Food and Drug Safety Evaluation;Available from: http://www.nifds.go.kr/toxinfo/Index.5. Joo CN, Choi SD. Sulfur metabolism in animal body -absorption of sulfur from intestine and its distribution-. Korean Biochem J. 1992. 25:397–402.6. Pappa A, Franco R, Schoneveld O, Galanis A, Sandaltzopoulos R, Panayiotidis MI. Sulfur-containing compounds in protecting against oxidant-mediated lung diseases. Curr Med Chem. 2007. 14:2590–2596.
Article7. Pratsel HG, Eigner UM, Weinert D, Limbach B. The analgesic efficacy of sulfur mud baths in treating rheumatic diseases of the soft tissues. A study using the double-blind control method. Vopr Kurortol Fizioter Lech Fiz Kult. 1992. (3):37–41.8. Jung E. Sulfur, oil and tar-oil baths in geriatrics. Z Krankenpfl. 1971. 64:230–232.9. Brown RG, Button GM, Smith JT. Changes in collagen metabolism caused by feeding diets low in inorganic sulfur. J Nutr. 1965. 87:228–232.
Article10. Button GM, Brown RG, Michels FG, Smith JT. Utilization of calcium and sodium sulfate by the rat. J Nutr. 1965. 87:211–216.
Article11. Kerr BJ, Weber TE, Ziemer CJ, Spence C, Cotta MA, Whitehead TR. Effect of dietary inorganic sulfur level on growth performance, fecal composition, and measures of inflammation and sulfate-reducing bacteria in the intestine of growing pigs. J Anim Sci. 2011. 89:426–437.
Article12. Anast C, Kennedy R, Volk G, Adamson L. In vitro studies of sulfate transport by the small intestine of the rat, rabbit, and hamster. J Lab Clin Med. 1965. 65:903–911.13. Sasse CE, Baker DH. Sulfur utilization by the chick with emphasis on the effect of inorganic sulfate on the cystine-methionine interrelationship. J Nutr. 1974. 104:244–251.
Article14. Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002. 7:22–44.15. Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL, Park JH. Conjugated linoleic acid downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. J Nutr. 2003. 133:2675–2681.
Article16. Lee HS, Seo EY, Kim WK. Resveratrol induces apoptosis in SW480 human colon cancer cell lines. Food Sci Biotechnol. 2004. 13:80–84.17. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003. 284:G996–G1005.18. Kong CS, Bak SS, Rhee SH, Rho CW, Kim NK. Fermentation properties of young radish Kimchi prepared using young radish cultivated in the soil containing sulfur and it's inhibitory effect on the growth of AGS human gastric adenocarcinoma cells. J Korean Soc Food Sci Nutr. 2006. 35:158–163.
Article19. Bak SS, Kong CS, Rhee SH, Rho CW, Kim NK, Choi KL, Park KY. Effect of sulfur enriched young radish Kimchi on the induction of apoptosis in HT-29 human colon cancer cells. J Food Sci Nutr. 2006. 11:184–190.
Article20. Choi GH, Kim CH. Growth inhibition of extract from sulfur fed duck carcass against various cancer cell lines. J Korean Soc Food Sci Anim Resour. 2002. 22:348–351.21. Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000. 60:1426–1433.22. Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004. 25:83–90.
Article23. Takeuchi K, Ito F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010. 277:316–326.
Article24. Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003. 12:541–552.
Article25. Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006. 7:505–516.
Article26. Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992. 89:10578–10582.
Article27. Han SW, Choi YY, Woo HD, Sohn DM, Bae SH, Gang GH, Kim SY, Baek MJ, Lim CW, Lee MS, Kim CH, Lee MH, Rho JH, Cho HD, Oh MH, Kim EH, Cho MS. Expression of HER-2/neu and paxillin in ductal carcinoma in situ, invasive ductal carcinoma with ductal carcinoma in situ and mucinous carcinoma. J Breast Cancer. 2008. 11:109–115.
Article28. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004. 101:13306–13311.
Article29. Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003. 9:2316–2326.30. Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998. 282:1318–1321.
Article31. del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997. 278:687–689.
Article32. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999. 96:857–868.
Article33. Takeuchi K, Ito F. Suppression of adriamycin-induced apoptosis by sustained activation of the phosphatidylinositol-3'-OH kinase-Akt pathway. J Biol Chem. 2004. 279:892–900.
Article34. Binder C, Marx D, Binder L, Schauer A, Hiddemann W. Expression of Bax in relation to Bcl-2 and other predictive parameters in breast cancer. Ann Oncol. 1996. 7:129–133.
Article35. Keane MM, Ettenberg SA, Lowrey GA, Russell EK, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res. 1996. 56:4791–4798.36. Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997. 139:1281–1292.
Article37. Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004. 279:21085–21095.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inorganic sulfur reduces the motility and invasion of MDA-MB-231 human breast cancer cells
- Effect of[6]-Gingerol on Inhibition of Cell Proliferation in MDA-MB-231 Human Breast Cancer Cells
- Radish (Raphanus sativus L. leaf) ethanol extract inhibits protein and mRNA expression of ErbB2 and ErbB3 in MDA-MB-231 human breast cancer cells
- Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines
- Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells