Clin Exp Vaccine Res.
2017 Jul;6(2):156-159. 10.7774/cevr.2017.6.2.156.
Microneedles: quick and easy delivery methods of vaccines
- Affiliations
-
- 1Interpark Bio-Convergence Center, Seoul, Korea. jooyoung.kim@imarketkorea.com
- KMID: 2391576
- DOI: http://doi.org/10.7774/cevr.2017.6.2.156
Abstract
- Vaccination is the most efficient method for infectious disease prevention. Parenteral injections such as intramuscular, intradermal, and subcutaneous injections have several advantages in vaccine delivery, but there are many drawbacks. Thus, the development of a new vaccine delivery system has long been required. Recently, microneedles have been attracting attention as new vaccination tools. Microneedle is a highly effective transdermal vaccine delivery method due to its mechanism of action, painlessness, and ease of use. Here, we summarized the characteristics of microneedles and the possibilities as a new vaccine delivery route.
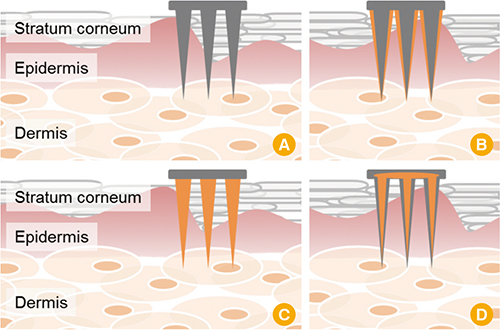
Figure
Reference
-
1. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011; 11:865–872.
Article2. Strugnell R, Zepp F, Cunningham A, Tantawichien T. Vaccine antigens. Perspect Vaccinol. 2011; 1:61–88.
Article3. Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009; 183:6883–6892.
Article4. Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010; 8:62–73.
Article5. Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005; 5:905–916.
Article6. Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006; 6:148–158.
Article7. Shim BS, Choi YK, Yun CH, et al. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PLoS One. 2011; 6:e27953.
Article8. Chandrashekar NS, Shobha Rani RH. Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian J Pharm Sci. 2008; 70:94–96.
Article9. Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008; 26:1261–1268.
Article10. Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004; 3:115–124.
Article11. Suh H, Shin J, Kim YC. Microneedle patches for vaccine delivery. Clin Exp Vaccine Res. 2014; 3:42–49.
Article12. Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009; 333:369–393.
Article13. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012; 64:1547–1568.
Article14. Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Hum Vaccin Immunother. 2016; 12:2975–2983.
Article15. Skountzou I, Compans RW. Skin immunization with influenza vaccines. Curr Top Microbiol Immunol. 2015; 386:343–369.
Article16. Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. 2017; 8:177–200.
Article