J Gastric Cancer.
2015 Dec;15(4):223-230. 10.5230/jgc.2015.15.4.223.
Association between Chemotherapy-Response Assays and Subsets of Tumor-Infiltrating Lymphocytes in Gastric Cancer: A Pilot Study
- Affiliations
-
- 1Department of Surgery, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea. cairus@yuhs.ac
- 2Translational Xenotransplantation Research Center, Seoul, Korea.
- 3Department of Microbiology and Immunology, Seoul, Korea.
- 4Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Open NBI Convergence Technology Research Laboratory, Severance Hospital, Yonsei University Health System, Seoul, Korea.
- KMID: 2391556
- DOI: http://doi.org/10.5230/jgc.2015.15.4.223
Abstract
- PURPOSE
The purpose of this pilot study was to evaluate the association between adenosine triphosphate-based chemotherapy response assays (ATP-CRAs) and subsets of tumor infiltrating lymphocytes (TILs) in gastric cancer.
MATERIALS AND METHODS
In total, 15 gastric cancer tissue samples were obtained from gastrectomies performed between February 2007 and January 2011. Chemotherapy response assays were performed on tumor cells from these samples using 11 chemotherapeutic agents, including etoposide, doxorubicin, epirubicin, mitomycin, 5-fluorouracil (5-FU), oxaliplatin, irinotecan, docetaxel, paclitaxel, methotrexate, and cisplatin. TILs in the tissue samples were evaluated using antibodies specific for CD3, CD4, CD8, Foxp3, and Granzyme B.
RESULTS
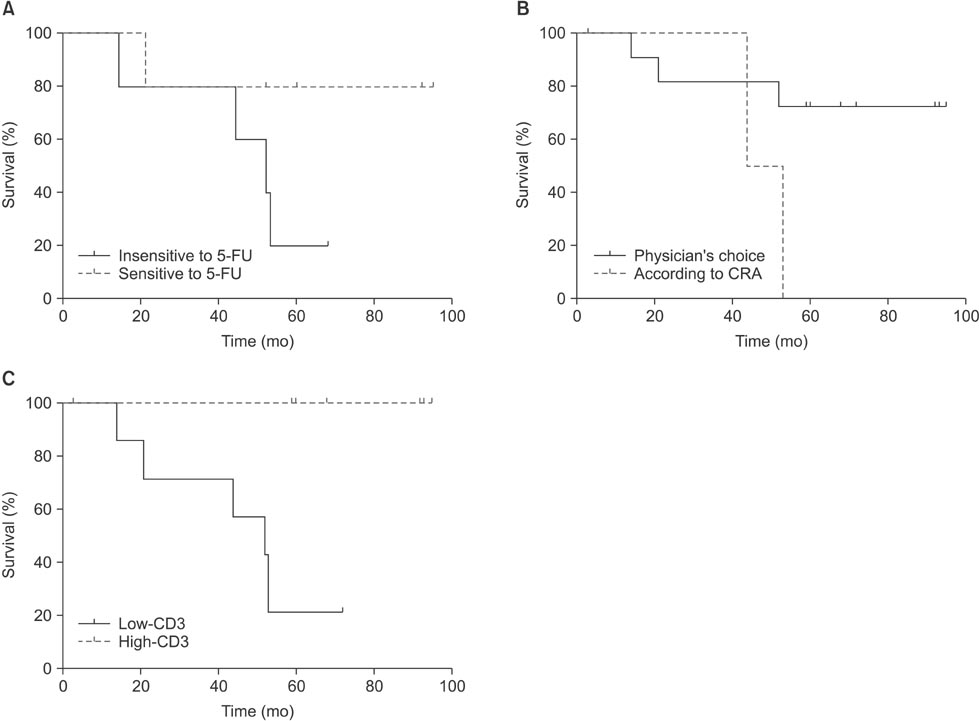
The highest cancer cell death rates were induced by etoposide (44.8%), 5-FU (43.1%), and mitomycin (39.9%). Samples from 10 patients who were treated with 5-FU were divided into 5-FU-sensitive and -insensitive groups according to median cell death rate. No difference was observed in survival between the two groups (P=0.216). Only two patients were treated with a chemotherapeutic agent determined by an ATP-CRA and there was no significant difference in overall survival compared with that of patients treated with their physician's choice of chemotherapeutic agent (P=0.105). However, a high number of CD3 TILs was a favorable prognostic factor (P=0.008). Pearson's correlation analyses showed no association between cancer cell death rates in response to chemotherapeutic agents and subsets of TILs.
CONCLUSIONS
Cancer cell death rates in response to specific chemotherapeutic agents were not significantly associated with the distribution of TIL subsets.
Keyword
MeSH Terms
-
Adenosine
Adenosine Triphosphate
Antibodies
Cell Death
Cisplatin
Doxorubicin
Drug Screening Assays, Antitumor
Drug Therapy
Epirubicin
Etoposide
Fluorouracil
Gastrectomy
Granzymes
Humans
Lymphocytes, Tumor-Infiltrating*
Methotrexate
Mitomycin
Paclitaxel
Pilot Projects*
Stomach Neoplasms*
Adenosine
Adenosine Triphosphate
Antibodies
Cisplatin
Doxorubicin
Epirubicin
Etoposide
Fluorouracil
Granzymes
Methotrexate
Mitomycin
Paclitaxel
Figure
Cited by 1 articles
-
Biomarkers for Evaluating the Inflammation Status in Patients with Cancer
Ali Guner, Hyoung-Il Kim
J Gastric Cancer. 2019;19(3):254-277. doi: 10.5230/jgc.2019.19.e29.
Reference
-
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.2. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–730.3. Macdonald JS, Fleming TR, Peterson RF, Berenberg JL, Mc-Clure S, Chapman RA, et al. Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma: a Southwest Oncology Group study. Ann Surg Oncol. 1995; 2:488–494.4. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000; 87:236–242.5. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396.6. Park S, Woo Y, Kim H, Lee YC, Choi S, Hyung WJ, et al. In Vitro adenosine triphosphate based chemotherapy response assay in gastric cancer. J Gastric Cancer. 2010; 10:155–161.7. Park JY, Kim YS, Bang S, Hyung WJ, Noh SH, Choi SH, et al. ATP-based chemotherapy response assay in patients with unresectable gastric cancer. Oncology. 2007; 73:439–440.8. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013; 31:860–867.9. Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010; 28:105–113.10. Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011; 6:49.11. Liu H, Zhang T, Ye J, Li H, Huang J, Li X, et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012; 61:1849–1856.12. Zingg U, Montani M, Frey DM, Dirnhofer S, Went P, Oertli D. Influence of neoadjuvant radio-chemotherapy on tumor-infiltrating lymphocytes in squamous esophageal cancer. Eur J Surg Oncol. 2009; 35:1268–1272.13. West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011; 13:R126.14. Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008; 14:1413–1417.15. Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rödel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014; 110:501–509.16. Kim HI, Kim H, Cho HW, Kim SY, Song KJ, Hyung WJ, et al. The ratio of intra-tumoral regulatory T cells (Foxp3+)/helper T cells (CD4+) is a prognostic factor and associated with recurrence pattern in gastric cardia cancer. J Surg Oncol. 2011; 104:728–733.17. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011; 105:93–103.18. Minamoto T, Mai M, Watanabe K, Ooi A, Kitamura T, Takahashi Y, et al. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer. 1990; 66:945–952.19. Kang SM, Park MS, Chang J, Kim SK, Kim H, Shin DH, et al. A feasibility study of adenosine triphosphate-based chemotherapy response assay (ATP-CRA) as a chemosensitivity test for lung cancer. Cancer Res Treat. 2005; 37:223–227.20. Weisenthal LM, Dill PL, Finklestein JZ, Duarte TE, Baker JA, Moran EM. Laboratory detection of primary and acquired drug resistance in human lymphatic neoplasms. Cancer Treat Rep. 1986; 70:1283–1295.21. Bird MC, Bosanquet AG, Gilby ED. In vitro determination of tumour chemosensitivity in haematological malignancies. Hematol Oncol. 1985; 3:1–10.22. Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007; 117:1130–1136.23. Burnette B, Weichselbaum RR. Radiation as an immune modulator. Semin Radiat Oncol. 2013; 23:273–280.24. Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001; 7:3025–3030.25. Halama N, Michel S, Kloor M, Zoernig I, Pommerencke T, von Knebel Doeberitz M, et al. The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun. 2009; 9:1.26. Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011; 71:5670–5677.27. de Kruijf EM, van Nes JG, Sajet A, Tummers QR, Putter H, Osanto S, et al. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res. 2010; 16:1272–1280.28. Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008; 118:1991–2001.29. Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008; 14:2413–2420.30. Lake RA, Robinson BW. Immunotherapy and chemotherapy: a practical partnership. Nat Rev Cancer. 2005; 5:397–405.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Tumor-infiltrating Lymphocytes in Breast Cancer after Neoadjuvant Chemotherapy
- The immunological characteristics of tumor infiltrating lymphocytes and tumor draining lymph node lymphocytes in advanced stomach cancer
- The Study for the Postoperative Changes of Peripheral Lymphocytes and their Subsets as Immune Function in Advanced Gastric Cancer
- Study of the Expression of FasL and of Apoptosis in Gastric Epithelial Dysplasia and Gastric Adenocarcinomas
- Current Issues and Clinical Evidence in Tumor-Infiltrating Lymphocytes in Breast Cancer