Ann Rehabil Med.
2012 Oct;36(5):596-608.
The Effect of Electric Cortical Stimulation after Focal Traumatic Brain Injury in Rats
- Affiliations
-
- 1Department of Rehabilitation Medicine, Presbyterian Medical Center, Jeonju 560-750, Korea. littlemicky@hanmail.net
- 2Department of NeuroSurgery, Presbyterian Medical Center, Jeonju 560-750, Korea.
- 3Department of Rehabilitation Medicine, School of Medicine, Chungnam National University, Daejeon 301-721, Korea.
Abstract
OBJECTIVE
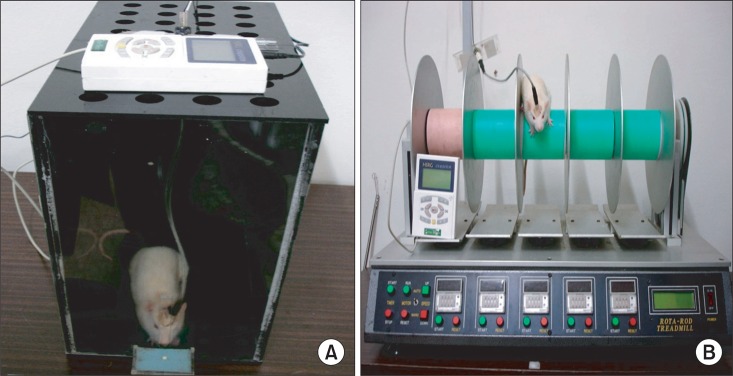
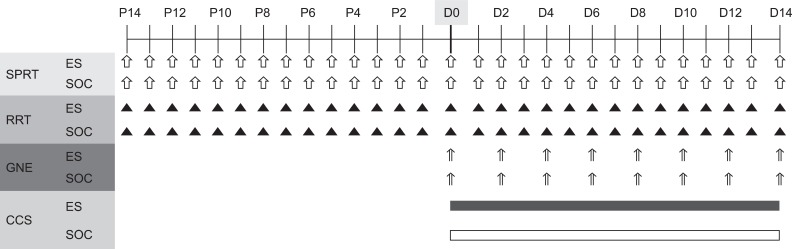
To evaluate the effects of electric cortical stimulation in the experimentally induced focal traumatic brain injury (TBI) rat model on motor recovery and plasticity of the injured brain. METHOD: Twenty male Sprague-Dawley rats were pre-trained on a single pellet reaching task (SPRT) and on a Rotarod task (RRT) for 14 days. Then, the TBI model was induced by a weight drop device (40 g in weight, 25 cm in height) on the dominant motor cortex, and the electrode was implanted over the perilesional cortical surface. All rats were divided into two groups as follows: Electrical stimulation (ES) group with anodal continuous stimulation (50 Hz and 194 micros duration) or Sham-operated control (SOC) group with no electrical stimulation. The rats were trained SPRT and RRT for 14 days for rehabilitation and measured Garcia's neurologic examination. Histopathological and immunostaining evaluations were performed after the experiment.
RESULTS
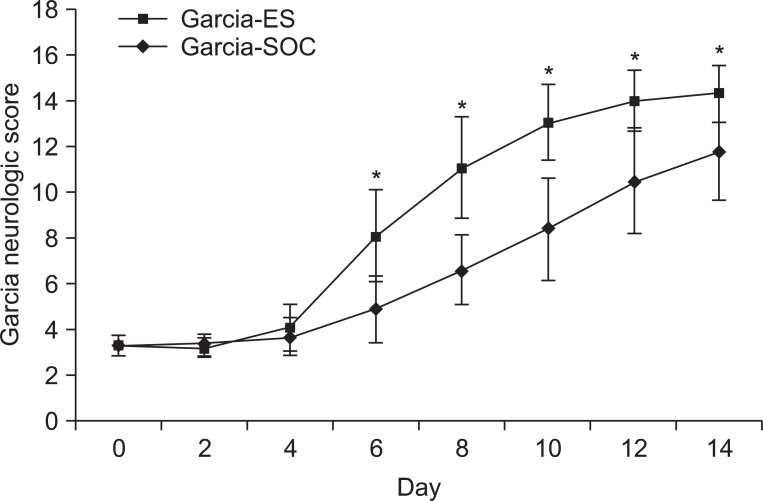
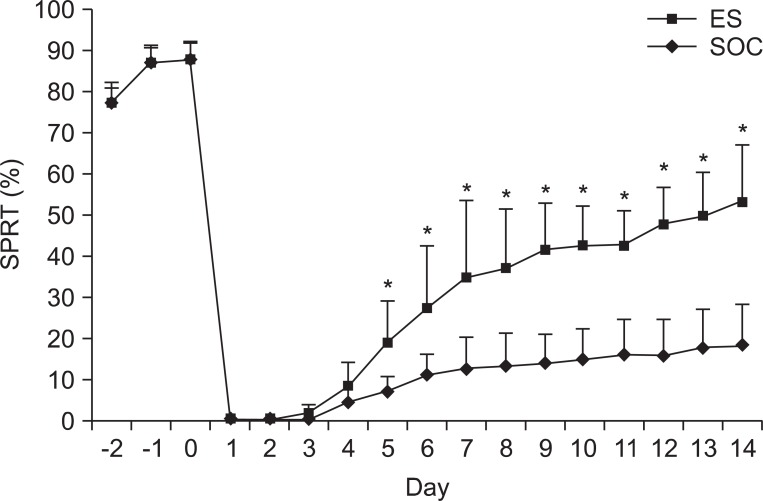
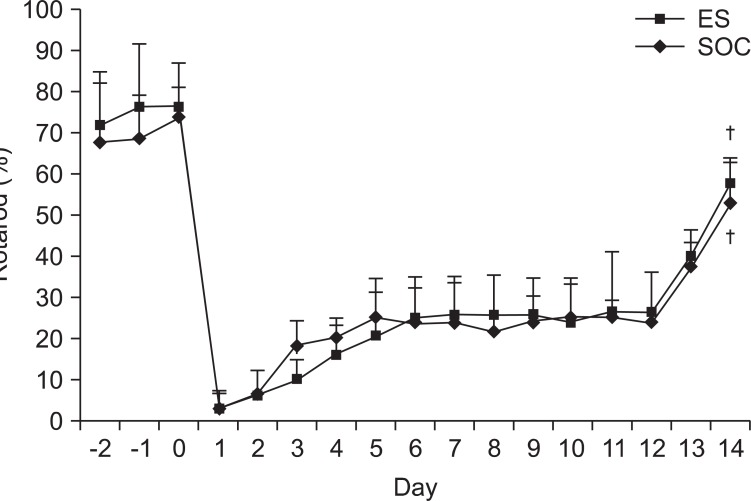
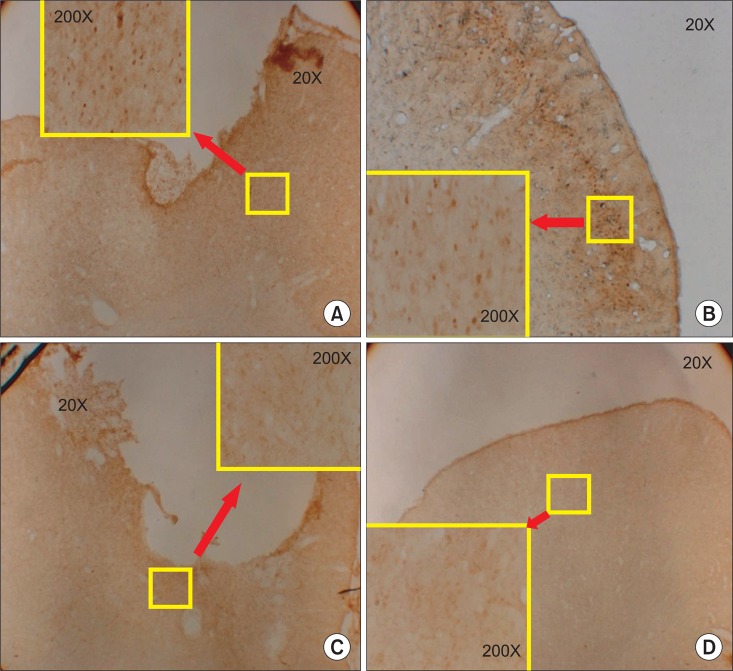
There were no differences in the slice number in the histological analysis. Garcia's neurologic scores & SPRT were significantly increased in the ES group (p<0.05), yet, there was no difference in RRT in both groups. The ES group showed more expression of c-Fos around the brain injured area than the SOC group.
CONCLUSION
Electric cortical stimulation with rehabilitation is considered to be one of the trial methods for motor recovery in TBI. However, more studies should be conducted for the TBI model in order to establish better stimulation methods.
MeSH Terms
Figure
Reference
-
1. Cifu DX, Kreutzer JS, Slaster DN, Tayor L. Braddom RL, editor. Rehabilitation after traumatic brain injury. Physical medicine and rehabilitation. 2006. 3rd ed. Philadelphia: Saunders;p. 1133–1174.2. Walsh V, Desmond JE, Pascual-Leone A. Manipulating brains. Behav Neurol. 2006; 17:131–134. PMID: 17148832.
Article3. Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007; 3:383–393. PMID: 17611487.
Article4. Alonso-Alonso M, Fregni F, Pascual-Leone A. Brain stimulation in poststroke rehabilitation. Cerebrovasc Dis. 2007; 24(Suppl 1):157–166. PMID: 17971652.
Article5. Pascual-Leone A. Disrupting the brain to guide plasticity and improve behavior. Prog Brain Res. 2006; 157:315–329. PMID: 17167918.
Article6. Harris-Love ML, Cohen LG. Noninvasive cortical stimulation in neurorehabilitation: a review. Arch Phys Med Rehabil. 2006; 87:S84–S93. PMID: 17140884.
Article7. Harvey RL, Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. Top Stroke Rehabil. 2007; 14:54–67. PMID: 18174116.
Article8. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien). 1991; 52:137–139. PMID: 1792954.
Article9. Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003; 25:789–793. PMID: 14669520.
Article10. Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003; 25:801–810. PMID: 14669522.
Article11. Katayama Y, Tsubokawa T, Yamamoto T. Chronic motor cortex stimulation for central deafferentation pain: experience with bulbar pain secondary to Wallenberg syndrome. Stereotact Funct Neurosurg. 1994; 62:295–299. PMID: 7631085.
Article12. Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003; 25:815–818. PMID: 14669524.
Article13. Brown JA, Lutsep H, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006; 58:464–473. PMID: 16528186.
Article14. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2007. 6th ed. London: Elsevier;p. 30–82.15. Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Response to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981; 211:67–77. PMID: 7225844.16. Ducker TB. Vinken PJ, Bruyn GW, editors. Experimental injury of the spinal cord. Handbook of clinical neurology. 1976. 1st ed. Amsterdam: North-Hollan Publishing Co.;p. 9–26.17. Yang CY, Moon SK, Song JH, Kim HS, Han EH, Kim TJ, Shin YI. The effect of continuous epidural electrical stimulation on synapse and neuronal cell in rat with focal ischemia. J Korean Acad Rehabil Med. 2008; 32:375–387.18. Vergara-Aragon P, Gonzalez CL, Whishaw IQ. A novel skilled-reaching impairment in paw supination on the "good" side of the hemi-Parkinson rat improved with rehabilitation. J Neurosci. 2003; 23:579–586. PMID: 12533618.
Article19. Moon SK, Yang CY, No SE, Kim EY, Lee S, Park SA, Oh GJ, Kim HI, Song JH, Lee MC, et al. Promotion of motor recovery by anodal continuous and low amplitude cortical stimulation in rat stroke model. Lab Anim Res. 2007; 23:25–30.20. Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol Sci. 1998; 19:59–66. PMID: 9550943.21. Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995; 26:627–663. PMID: 7709410.22. Cogiamanian F, Marceglia S, Rossi L, Torre ED, Priori A. Canavero S, editor. Transcranial magnectic and direct current stimulation: a primer. Textbook of therapeutic cortical stimulation. 2009. NewYork: Nova Science Publishers;p. 45–56.23. Sawaki L, Wu CW, Kaelin-Lang A, Cohen LG. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006; 37:246–247. PMID: 16322491.
Article24. Kreisel SH, Hennerici MG, Bazner H. Pathophysiology of stroke rehabilitation: the natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc Dis. 2007; 23:243–255. PMID: 17192704.
Article25. Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003; 25:780–788. PMID: 14669519.
Article26. Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003; 25:794–800. PMID: 14669521.
Article27. Moon SK, Shin YI, Kim HI, Kim HJ, Lee JO, Lee MC. Effect of prolonged cortical stimulation differs with size of infarct after sensorimotor cortical lesions in rats. Neurosci Lett. 2009; 460:152–155. PMID: 19450662.
Article28. Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma. 2003; 20:123–137. PMID: 12675967.
Article29. Park HK, Fernandez II, Dujovny M, Diaz FG. Experimental animal models of traumatic brain injury: medical and biomechanical mechanism. Crit Rev Neurosurg. 1999; 9:44–52. PMID: 9933368.
Article30. Gharbawie OA, Gonzalez CLR, Whishaw IQ. Skilled reaching impairment from the lateral frontal cortex component of middle cerebral artery stroke: a qualitative and quantitative comparison to focal motor cortex lesions in rat. Behav Brain Res. 2005; 156:125–137. PMID: 15474657.31. Whishaw IQ, Sarna JR, Pellis SM. Evidence for rodent-common and species-typical limb and digit use in eating, derived from a comparative analysis of ten rodent species. Behav Brain Res. 1998; 96:79–91. PMID: 9821545.
Article32. Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use dependent structural events in recovery of function. Adv Neurol. 1997; 73:229–238. PMID: 8959217.33. Gonzalez CL, Kolb B. A comparison of different models of stroke on behavior and brain morphology. Eur J Neurosci. 2003; 18:1950–1962. PMID: 14622227.34. Whishaw IQ, Kolb B. "Stick out your tongue": tongue protrusion in neocortex and hypothalamus damaged rats. Physiol Behav. 1983; 30:471–480. PMID: 6867143.35. Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 2004; 1019:47–54. PMID: 15306237.
Article36. Kim DY, Park SH, Lee SU, Choi DH, Park HW, Paek SH, Shin HY, Kim EY, Park SP, Lim JH. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci Res. 2007; 58:164–175. PMID: 17408791.
Article37. Kim HS, Shin YI, Kim HI, Moon SK, Lee JO, Moon BS, Lee MC. Relevance of behavioral test in the photothrombotic stroke rat model. J Korean Acad Rehabil Med. 2006; 30:135–141.38. Gonzalez CLR, Kolb B. A comparison of different models of stroke on behavior and brain morphology. Eur J Neurosci. 2003; 18:1950–1962. PMID: 14622227.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Electric Cortical Stimulation (ECS) and Transcranial Direct Current Stimulation (tDCS) on Rats With a Traumatic Brain Injury
- Safety Review for Clinical Application of Repetitive Transcranial Magnetic Stimulation
- Effect of Vagus Nerve Stimulation in Post-Traumatic Epilepsy and Failed Epilepsy Surgery : Preliminary Report
- The effect of local electric stimulation on the survival of the random pattern skin flaps in rats
- Movement Disorders after Traumatic Brain Injury