Korean J Gastroenterol.
2017 Aug;70(2):96-102. 10.4166/kjg.2017.70.2.96.
Clinical Interpretation of Elevated CA 19-9 Levels in Obstructive Jaundice Following Benign and Malignant Pancreatobiliary Disease
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 2Division of Gastroenterology, Department of Internal Medicine, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. drjtj@paik.ac.kr
- KMID: 2391367
- DOI: http://doi.org/10.4166/kjg.2017.70.2.96
Abstract
- BACKGROUND/AIMS
Elevated carbohydrate antigen (CA) 19-9 level may be unable to differentiate between benign and malignant pancreatobiliary disease with obstructive jaundice. The study aims to determine the clinical interpretation and the diagnostic value of CA 19-9 level in pancreatobiliary diseases with coexistent obstructive jaundice.
METHODS
We retrospectively reviewed the data of 981 patients who underwent biliary drainage due to obstructive jaundice following pancreatobiliary disease at Sanggye Paik Hospital for 5 years. 114 patients with serial follow-up data for CA 19-9 level were included in this study (80 patients with malignancy and 34 patients with benign diseases). We compared the levels of CA 19-9 levels and the biochemical value before and after biliary drainage.
RESULTS
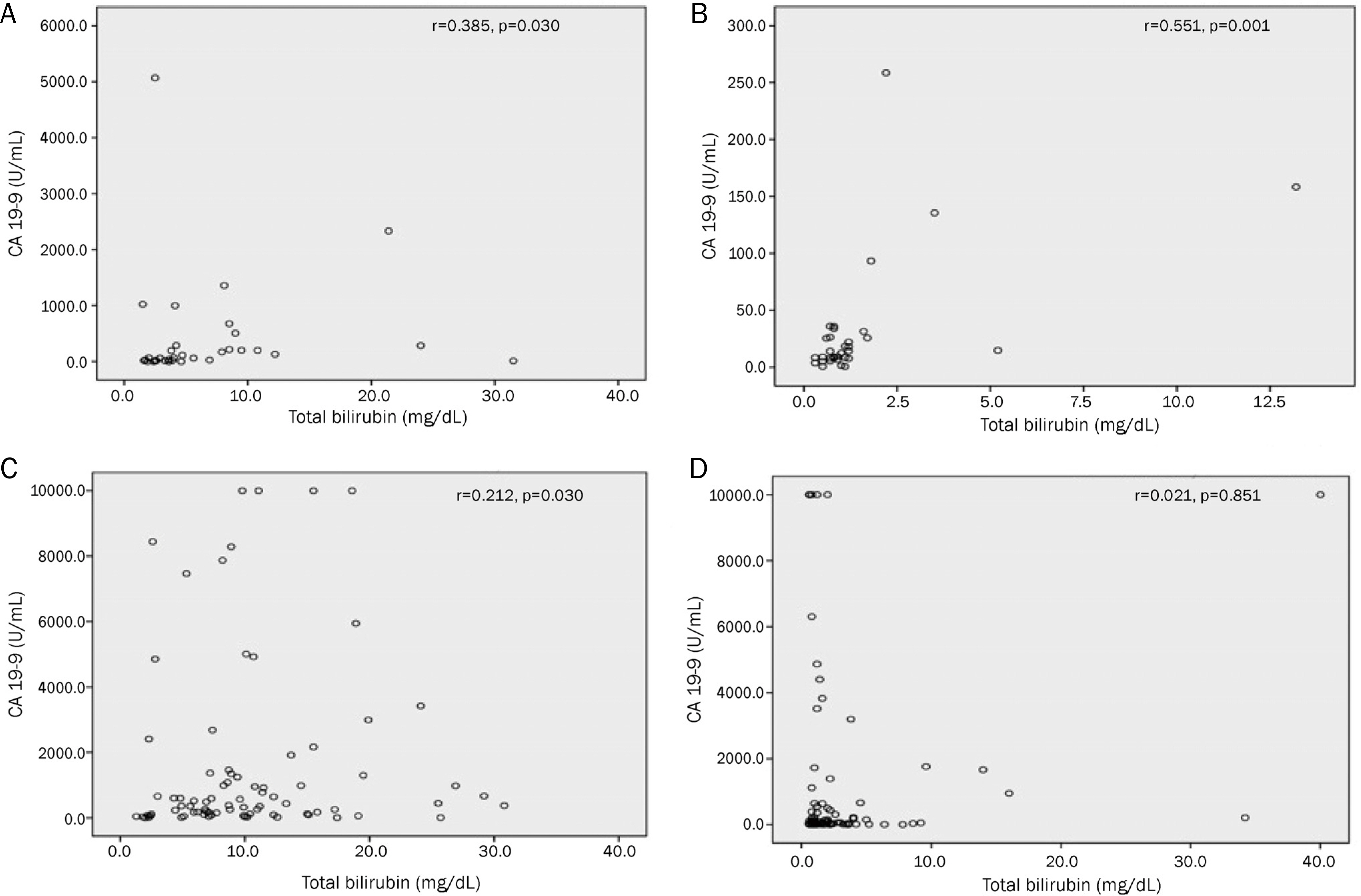
The rate of CA 19-9 elevation (>37 U/mL) was significantly different between the benign group and the malignant group (59% vs. 90%, p=0.001). Despite the decrease in serum bilirubin after biliary drainage, CA 19-9 levels remained elevated in 12% of patients in the benign group and in 63% of patients in the malignant group (p<0.001). Finally, 12% of patients in the benign group turned out to have malignant disease. A receiver operating characteristic analysis provided a cut-off value of 38 U/mL for differentiating benign disease from malignant disease after biliary drainage (area under curve, 0.787; 95% confidence interval, 0.703 to 0.871; sensitivity, 62%; specificity, 88%).
CONCLUSIONS
This study suggested that we should consider the possibility of malignant causes if the CA 19-9 levels remain high or are more than 38 U/mL after resolution of biliary obstruction.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Clinical Relevance of Tumor Marker CA 19-9
Yoon Suk Lee
Korean J Gastroenterol. 2017;70(2):61-63. doi: 10.4166/kjg.2017.70.2.61.
Reference
-
References
1. Albert MB, Steinberg WM, Henry JP. Elevated serum levels of abdominal marker CA19–9 in acute cholangitis. Dig Dis Sci. 1988; 33:1223–1225.2. Richter JM, Christensen MR, Rustgi AK, Silverstein MD. The abdominal utility of the Ca19–9 radioimmunoassay for the diagnosis of pancreatic cancer presenting as pain or weight loss. A cost-effectiveness analysis. Arch Intern Med. 1989; 149:2292–2297.3. Paganuzzi M, Onetto M, Marroni P, et al. CA 19–9 and CA 50 in benign and malignant pancreatic and biliary diseases. Cancer. 1988; 61:2100–2108.
Article4. Kim HJ, Kim MH, Myung SJ, et al. A new strategy for the application of CA19–9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol. 1999; 94:1941–1946.
Article5. Goonetilleke KS, Siriwardena AK. Systematic review of abdominal antigen (CA 19–9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007; 33:266–270.6. Kang CM, Kim JY, Choi GH, et al. The use of adjusted preoperative CA 19–9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007; 140:31–35.
Article7. Ong SL, Sachdeva A, Garcea G, et al. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci. 2008; 53:3213–3217.
Article8. Marrelli D, Caruso S, Pedrazzani C, et al. CA19–9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009; 198:333–339.
Article9. Morris-Stiff G, Teli M, Jardine N, Puntis MC. CA19–9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat Dis Int. 2009; 8:620–626.10. La Greca G, Sofia M, Lombardo R, et al. Adjusting CA19–9 values to predict malignancy in obstructive jaundice: influence of abdominal and C-reactive protein. World J Gastroenterol. 2012; 18:4150–4155.11. Park JK, Paik WH, Ryu JK, et al. Clinical significance and revisiting the meaning of CA 19–9 blood level before and after the abdominal of pancreatic ductal adenocarcinoma: analysis of 1,446 patients from the pancreatic cancer cohort in a single institution. PLoS One. 2013; 8:e78977. eCollection 2013.12. Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and abdominal biomarkers in pancreatic cancer. J Surg Oncol. 2013; 107:15–22.13. Le N, Sund M, Vinci A. GEMS collaborating group of Pancreas 2000. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis. 2016; 48:223–230.
Article14. Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19–9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000; 95:204–207.
Article15. Katsanos KH, Kitsanou M, Christodoulou DK, Tsianos EV. High CA 19–9 levels in benign biliary tract diseases. Report of four cases and review of the literature. Eur J Intern Med. 2002; 13:132–135.16. Lowe D, Lee J, Schade R, Chaudhary A. Patient with markedly abdominal CA 19–9 not associated with malignancy. South Med J. 2006; 99:306–308.17. Sanchez M, Gomes H, Marcus EN. Elevated CA 19–9 levels in a patient with mirizzi syndrome: case report. South Med J. 2006; 99:160–163.
Article18. Marcouizos G, Ignatiadou E, Papanikolaou GE, Ziogas D, Fatouros M. Highly elevated serum levels of CA 19–9 in choledocholithiasis: a case report. Cases J. 2009; 2:6662.
Article19. Bertino G, Ardiri AM, Calvagno GS, et al. Carbohydrate 19.9 abdominal serum levels in liver disease. Biomed Res Int. 2013; 2013:531640.20. Shin JY, Yoo SJ, Park BM, Jung SS, Kim JO, Lee JE. Extremely abdominald serum carbohydrate antigen 19–9 levels caused by new or resistant infections to previous antibiotics in chronic lung diseases. Tuberc Respir Dis (Seoul). 2013; 75:125–127.21. Su SB, Qin SY, Chen W, Luo W, Jiang HX. Carbohydrate antigen 19–9 for differential diagnosis of pancreatic carcinoma and chronic pancreatitis. World J Gastroenterol. 2015; 21:4323–4333.
Article22. Akimoto S, Banshodani M, Nishihara M, et al. Acute cholecystitis with significantly elevated levels of serum carbohydrate antigen 19–9. Case Rep Gastroenterol. 2016; 10:410–416.
Article23. Hong JY, Jang SH, Kim SY, et al. Elevated serum CA 19–9 levels in patients with pulmonary nontuberculous mycobacterial disease. Braz J Infect Dis. 2016; 20:26–32.
Article24. Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19–9, and CA 72–4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001; 181:16–19.
Article25. Stiksma J, Grootendorst DC, van der Linden PW. CA 19–9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014; 13:239–244.26. Scarpa M, Noaro G, Saadeh L, et al. Esophageal cancer abdominal: preoperative CA19.9 and CEA serum levels may identify occult advanced adenocarcinoma. World J Surg. 2015; 39:424–432.27. Song YX, Huang XZ, Gao P, et al. Clinicopathologic and prognostic value of serum carbohydrate antigen 19–9 in gastric cancer: a metaanalysis. Dis Markers. 2015; 2015:549843.
Article28. Duraker N, Hot S, Polat Y, Höbek A, Gençler N, Urhan N. CEA, CA 19–9, and CA 125 in the differential diagnosis of benign and abdominal pancreatic diseases with or without jaundice. J Surg Oncol. 2007; 95:142–147.29. Madonia S, Aragona E, Maisano S, et al. CA 19–9 to rule out abdominal or biliary cancer among patients with cholestasis: an unsuitable test? Dig Dis Sci. 2007; 52:1125–1127.30. Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979; 5:957–971.
Article31. Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19–9 and lewis antigens in pancreatic cancer. Cancer Res. 1987; 47:5501–5503.32. Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19–9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000; 26:474–479.
Article33. Mortenson MM, Katz MH, Tamm EP, et al. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008; 196:100–113.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Updates of Immunoglobulin G4-related Pancreatobiliary Disease
- Approach to the Patients with Elevated CA 19-9
- A Comparative Study on Serum Immunoglobulin and Tumor Marker Levels in the Patients with Autoimmune Pancreatitis and Pancreatobiliary Malignancies
- An Immunohistochemical Study of CA 125, CA 19-9, and CA 15-3 in Ovarian Epithelial Tumors
- Obstructive Jaundice