Allergy Asthma Immunol Res.
2016 May;8(3):206-215. 10.4168/aair.2016.8.3.206.
Flagellin Modulates the Function of Invariant NKT Cells From Patients With Asthma via Dendritic Cells
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School & Hospital, Gwangju, Korea. yikoh@chonnam.ac.kr
- 2Clinical Vaccine R&D Center, Department of Microbiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2391040
- DOI: http://doi.org/10.4168/aair.2016.8.3.206
Abstract
- PURPOSE
Invariant natural killer T (iNKT) cells play a critical role in the pathogenesis of asthma. We previously reported the association between circulating Th2-like iNKT cells and lung function in asthma patients and the suppressive effect of Toll-like receptor 5 ligand flagellin B (FlaB) on asthmatic in a mouse model. Thus, we investigated whether FlaB modulates the function of circulating iNKT cells in asthmatic patients.
METHODS
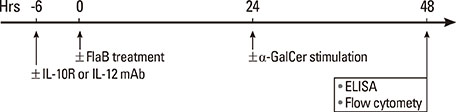
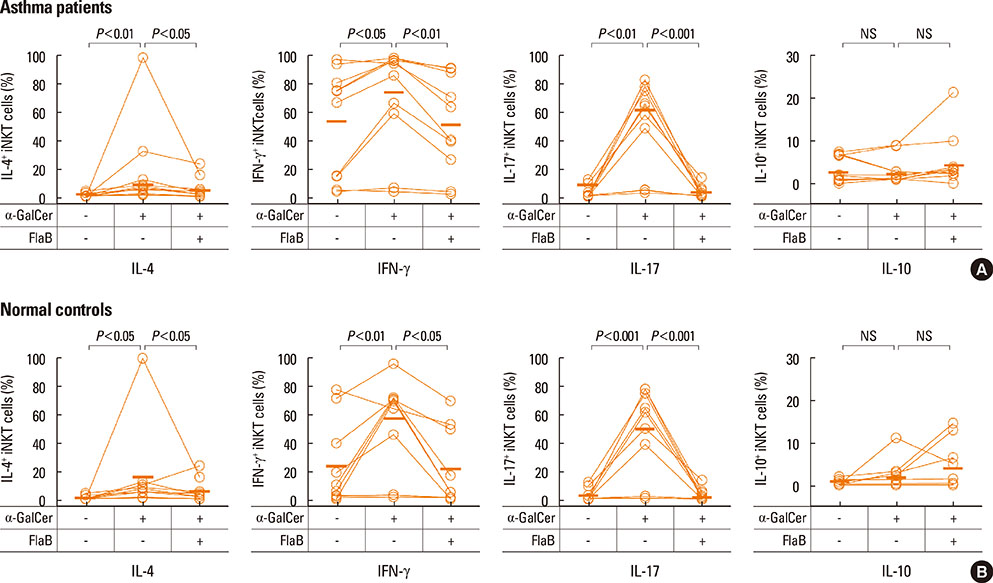
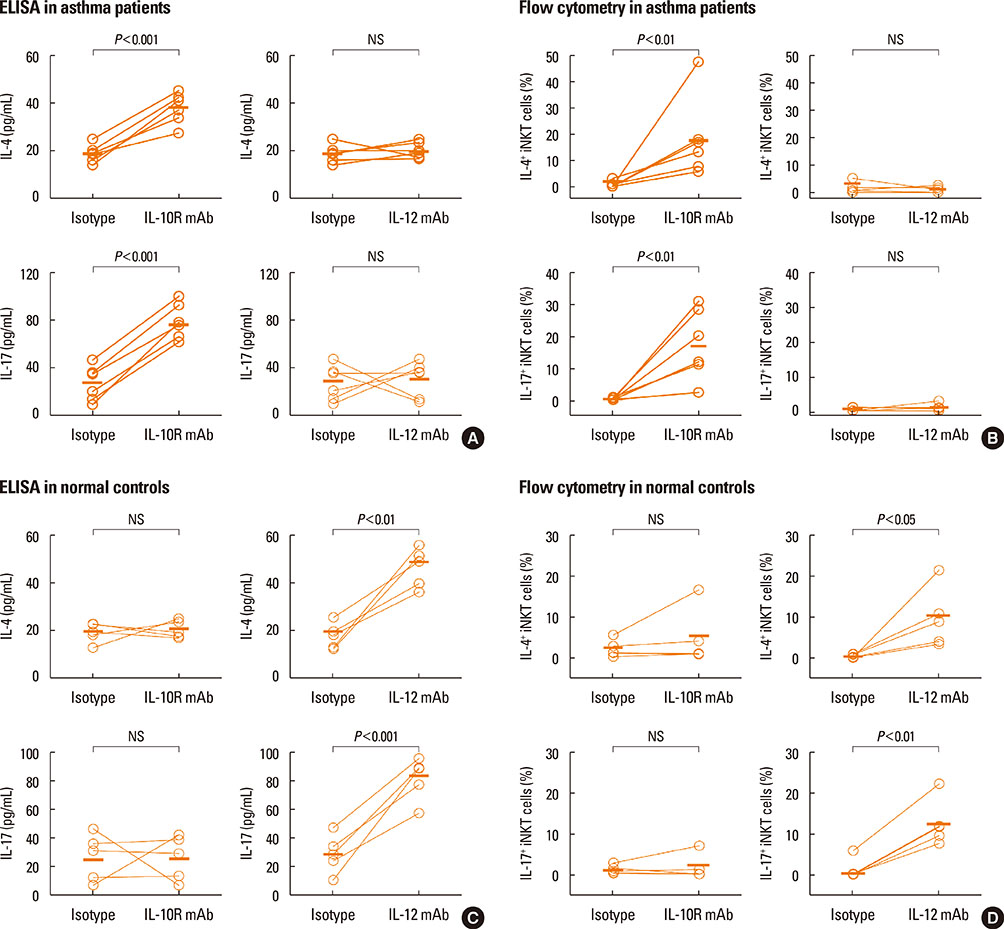
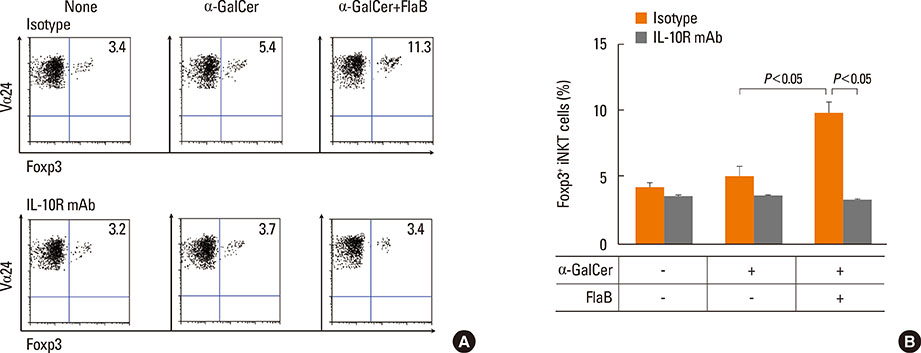
Peripheral blood mononuclear cells (PBMCs) were treated with FlaB, and the secreted and intracellular cytokines of iNKT cells were evaluated by using ELISA and flow cytometry, respectively, following stimulation with alpha-galactosylceramide. Foxp3+ iNKT cells were also measured. To determine the effect of FlaB-treated dendritic cells (DCs) on iNKT cells, we co-cultured CD14+ monocyte-derived DCs and T cells from patients with house dust mite-sensitive asthma and analyzed intracellular cytokines in iNKT cells.
RESULTS
A reduction of IL-4 and IL-17 production by iNKT cells in PBMCs after FlaB treatment was alleviated following blocking of IL-10 signaling. A decrease in the frequencies of IL-4+ and IL-17+ iNKT cells by FlaB-treated DCs was reversed after blocking of IL-10 signaling. Simultaneously, an increase in Foxp3+ iNKT cells induced by FlaB treatment disappeared after blocking of IL-10.
CONCLUSIONS
FlaB may inhibit Th2- and Th17-like iNKT cells and induce Foxp3+ iNKT cells by DCs via an IL-10-dependent mechanism in asthmatic patients. In patients with a specific asthma phenotype associated with iNKT cells, FlaB may be an effective immunomodulator for iNKT cell-targeted immunotherapy.
Keyword
MeSH Terms
-
Animals
Asthma*
Cytokines
Dendritic Cells*
Dust
Enzyme-Linked Immunosorbent Assay
Flagellin*
Flow Cytometry
Humans
Immunotherapy
Interleukin-10
Interleukin-17
Interleukin-4
Lung
Mice
Natural Killer T-Cells*
Phenotype
T-Lymphocytes
Toll-Like Receptor 5
Cytokines
Dust
Flagellin
Interleukin-10
Interleukin-17
Interleukin-4
Toll-Like Receptor 5
Figure
Cited by 1 articles
-
A Fusion Protein of Derp2 Allergen and Flagellin Suppresses Experimental Allergic Asthma
Wenzhi Tan, Jin Hai Zheng, Tra-My Nu Duong, Young-Il Koh, Shee Eun Lee, Joon Haeng Rhee
Allergy Asthma Immunol Res. 2019;11(2):254-266. doi: 10.4168/aair.2019.11.2.254.
Reference
-
1. Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002; 2:557–568.2. Umetsu DT, DeKruyff RH. A role for natural killer T cells in asthma. Nat Rev Immunol. 2006; 6:953–958.3. Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003; 9:582–588.4. Koh YI, Shim JU, Lee JH, Chung IJ, Min JJ, Rhee JH, et al. Natural killer T cells are dispensable in the development of allergen-induced airway hyperresponsiveness, inflammation and remodelling in a mouse model of chronic asthma. Clin Exp Immunol. 2010; 161:159–170.5. Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006; 103:2782–2787.6. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008; 205:385–393.7. Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008; 14:633–640.8. Koh YI, Kim HY, Meyer EH, Pichavant M, Akbari O, Yasumi T, et al. Activation of nonclassical CD1d-restricted NK T cells induces airway hyperreactivity in beta 2-microglobulin-deficient mice. J Immunol. 2008; 181:4560–4569.9. Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlström J, Kronenberg M, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006; 354:1117–1129.10. Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:1410–1422.11. Koh YI, Shim JU. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol. 2010; 153:239–248.12. Koh YI, Shim JU, Wi J, Kwon YE. The role of natural killer T cells in the pathogenesis of acute exacerbation of human asthma. Int Arch Allergy Immunol. 2012; 158:131–141.13. Shim JU, Koh YI. Increased Th2-like invariant natural killer T cells in peripheral blood from patients with asthma. Allergy Asthma Immunol Res. 2014; 6:444–448.14. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013; 13:101–117.15. Lee SE, Koh YI, Kim MK, Kim YR, Kim SY, Nam JH, et al. Inhibition of airway allergic disease by co-administration of flagellin with allergen. J Clin Immunol. 2008; 28:157–165.16. Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006; 74:694–702.17. Schülke S, Burggraf M, Waibler Z, Wangorsch A, Wolfheimer S, Kalinke U, et al. A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. J Allergy Clin Immunol. 2011; 128:1340–1348.e12.18. Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J Immunol. 2009; 182:7539–7547.19. Shim JU, Rhee JH, Koh YI. TLR4, 5, and 9 agonists inhibit murine airway invariant natural killer T Cells in an IL-12-dependent manner. Allergy Asthma Immunol Res. 2012; 4:295–304.20. Nguyen CT, Hong SH, Sin JI, Vu HV, Jeong K, Cho KO, et al. Flagellin enhances tumor-specific CD8(+) T cell immune responses through TLR5 stimulation in a therapeutic cancer vaccine model. Vaccine. 2013; 31:3879–3887.21. Moreno M, Mol BM, von Mensdorff-Pouilly S, Verheijen RH, de Jong EC, von Blomberg BM, et al. Differential indirect activation of human invariant natural killer T cells by Toll-like receptor agonists. Immunotherapy. 2009; 1:557–570.22. Monteiro M, Almeida CF, Caridade M, Ribot JC, Duarte J, Agua-Doce A, et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-beta. J Immunol. 2010; 185:2157–2163.23. Koh YI, Choi IS, Lee JJ. Effects of cytokine milieu secreted by BCG-treated dendritic cells on allergen-specific Th immune response. J Korean Med Sci. 2004; 19:640–646.24. Schülke S, Waibler Z, Mende MS, Zoccatelli G, Vieths S, Toda M, et al. Fusion protein of TLR5-ligand and allergen potentiates activation and IL-10 secretion in murine myeloid DC. Mol Immunol. 2010; 48:341–350.25. Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001; 2:725–731.26. Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+ CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005; 202:1539–1547.27. Huang H, Dawicki W, Zhang X, Town J, Gordon JR. Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25-/loFoxp3- effector T cells. J Immunol. 2010; 185:5003–5010.28. Li X, Yang A, Huang H, Zhang X, Town J, Davis B, et al. Induction of type 2 T helper cell allergen tolerance by IL-10-differentiated regulatory dendritic cells. Am J Respir Cell Mol Biol. 2010; 42:190–199.29. Shim JU, Lee SE, Hwang W, Lee C, Park JW, Sohn JH, et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J Allergy Clin Immunol. 2016; 137:426–435.30. Kim YJ, Kim HJ, Kang MJ, Yu HS, Seo JH, Kim HY, et al. Bacillus Calmette-Guérin suppresses asthmatic responses via CD4(+)CD25(+) regulatory T cells and dendritic cells. Allergy Asthma Immunol Res. 2014; 6:201–207.31. La Cava A, Van Kaer L, Fu-Dong-Shi . CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006; 27:322–327.32. Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003; 63:4516–4520.33. Thorburn AN, Foster PS, Gibson PG, Hansbro PM. Components of Streptococcus pneumoniae suppress allergic airways disease and NKT cells by inducing regulatory T cells. J Immunol. 2012; 188:4611–4620.34. Moreira-Teixeira L, Resende M, Coffre M, Devergne O, Herbeuval JP, Hermine O, et al. Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells. J Immunol. 2011; 186:5758–5765.35. Engelmann P, Farkas K, Kis J, Richman G, Zhang Z, Liew CW, et al. Characterization of human invariant natural killer T cells expressing FoxP3. Int Immunol. 2011; 23:473–484.36. Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014; 124:3725–3740.37. Terhorst D, Kalali BN, Weidinger S, Illig T, Novak N, Ring J, et al. Monocyte-derived dendritic cells from highly atopic individuals are not impaired in their pro-inflammatory response to toll-like receptor ligands. Clin Exp Allergy. 2007; 37:381–390.38. Lun SW, Wong CK, Ko FW, Hui DS, Lam CW. Expression and functional analysis of toll-like receptors of peripheral blood cells in asthmatic patients: implication for immunopathological mechanism in asthma. J Clin Immunol. 2009; 29:330–342.39. Kupz A, Curtiss R 3rd, Bedoui S, Strugnell RA. In vivo IFN-gamma secretion by NK cells in response to Salmonella typhimurium requires NLRC4 inflammasomes. PLoS One. 2014; 9:e97418.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- B Cells Promote Th1- Skewed NKT Cell Response by CD1d-TCR Interaction
- TLR4, 5, and 9 Agonists Inhibit Murine Airway Invariant Natural Killer T Cells in an IL-12-Dependent Manner
- Natural killer T cell and pathophysiology of asthma
- Deficiencies of Circulating Mucosal-associated Invariant T Cells and Natural Killer T Cells in Patients with Acute Cholecystitis
- Comparison of Invariant NKT Cells with Conventional T Cells by Using Gene Set Enrichment Analysis (GSEA)