Allergy Asthma Immunol Res.
2016 May;8(3):181-190. 10.4168/aair.2016.8.3.181.
A Comprehensive Review of the Treatment of Atopic Eczema
- Affiliations
-
- 1Department of Dermatology, The Catholic University of Korea, Seoul, Korea. drchosh@hotmail.com
- 2Department of Dermatology, Korea University College of Medicine, Seoul, Korea. skin4u@korea.ac.kr
- KMID: 2391037
- DOI: http://doi.org/10.4168/aair.2016.8.3.181
Abstract
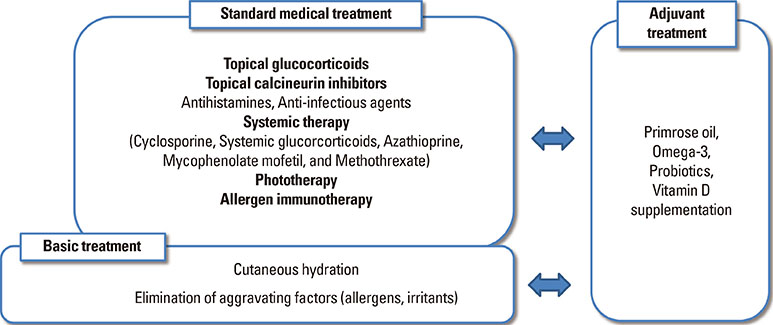
- Atopic eczema (AE) is a chronic, inflammatory skin disorder which usually develops in early childhood. In spite of intensive investigations, the causes of AE remain unclear, but are likely to be multifactorial in nature. Environmental factors or genetic-environmental interactions seem to play a key role in disease progression. Among various measures of AE managment, cutaneous hydration, which improves barrier function and relieve itchiness, may be helpful to reduce the need for topical steroid use and therefore should be used as a basic treatment. Avoiding aggravating factors is also a basic treatment of AE. Standard medical treatment with a pharmacologic approach may be necessary if basic treatment fails to control symptoms satisfactorily. Recently, more attention is given to a proactive therapeutic by regular intermittent application of low potency steroids or topical calcineurin inhibitors to prevent new flares. Furthermore, various targeted biologics are being introduced for AE control and are proposed as promising therapies. This paper provides a summary of the recent literature on the manangement of AE and a treatment guideline.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Current Management of Moderate-to-Severe Atopic Dermatitis: A Survey of Allergists, Pediatric Allergists and Dermatologists in Korea
Hye Yung Yum, Hyun Hee Kim, Hyun Jung Kim, Woo Kyung Kim, So-Yeon Lee, Kapsok Li, Dong Hun Lee,
Allergy Asthma Immunol Res. 2018;10(3):253-259. doi: 10.4168/aair.2018.10.3.253.
Reference
-
1. Williams HC, Strachan DP. The natural history of childhood eczema: observations from the British 1958 birth cohort study. Br J Dermatol. 1998; 139:834–839.2. Friedmann PS. The pathogenesis of atopic eczema. Hosp Med. 2002; 63:653–656.3. Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015; 7:101–105.4. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006; 38:441–446.5. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006; 368:733–743.6. von Mutius E. Gene-environment interactions in asthma. J Allergy Clin Immunol. 2009; 123:3–11.7. Lee JH, Lee HS, Park MR, Lee SW, Kim EH, Cho JB, et al. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol Res. 2014; 6:517–524.8. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009; 124:1251–1258.e23.9. Williams H, Robertson C, Stewart A, Aït-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999; 103:125–138.10. Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 1992; 17:385–391.11. Saito H. Much atopy about the skin: genome-wide molecular analysis of atopic eczema. Int Arch Allergy Immunol. 2005; 137:319–325.12. Asher MI, Weiland SK. ISAAC Steering Committee. The International Study of Asthma and Allergies in Childhood (ISAAC). Clin Exp Allergy. 1998; 28:Suppl 5. 52–66.13. Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008; 121:947–954.e15.14. Harrop J, Chinn S, Verlato G, Olivieri M, Norbäck D, Wjst M, et al. Eczema, atopy and allergen exposure in adults: a population-based study. Clin Exp Allergy. 2007; 37:526–535.15. Hanifin JM, Reed ML. Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007; 18:82–91.16. Grize L, Gassner M, Wüthrich B, Bringolf-Isler B, Takken-Sahli K, Sennhauser FH, et al. Trends in prevalence of asthma, allergic rhinitis and atopic dermatitis in 5-7-year old Swiss children from 1992 to 2001. Allergy. 2006; 61:556–562.17. Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011; 131:67–73.18. Saeki H, Iizuka H, Mori Y, Akasaka T, Takagi H, Kitajima Y, et al. Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br J Dermatol. 2005; 152:110–114.19. Saeki H, Oiso N, Honma M, Odajima H, Iizuka H, Kawada A, et al. Comparison of prevalence of atopic dermatitis in Japanese elementary schoolchildren between 2001/2002 and 2007/2008. J Dermatol. 2009; 36:512–514.20. Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, et al. Prevalences of symptoms of asthma and other allergic diseases in Korean children: a nationwide questionnaire survey. J Korean Med Sci. 2001; 16:155–164.21. Baek JO, Hong S, Son DK, Lee JR, Roh JY, Kwon HJ. Analysis of the prevalence of and risk factors for atopic dermatitis using an ISAAC questionnaire in 8,750 Korean children. Int Arch Allergy Immunol. 2013; 162:79–85.22. Krakowski AC, Eichenfield LF, Dohil MA. Management of atopic dermatitis in the pediatric population. Pediatrics. 2008; 122:812–824.23. Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009; 129:682–689.24. Gutman AB, Kligman AM, Sciacca J, James WD. Soak and smear: a standard technique revisited. Arch Dermatol. 2005; 141:1556–1559.25. Sher LG, Chang J, Patel IB, Balkrishnan R, Fleischer AB Jr. Relieving the pruritus of atopic dermatitis: a meta-analysis. Acta Derm Venereol. 2012; 92:455–461.26. Anderson PC, Dinulos JG. Are the new moisturizers more effective? Curr Opin Pediatr. 2009; 21:486–490.27. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014; 71:116–132.28. Lee JH, Jung KE, Lee YB, Kim JE, Kim HS, Lee KH, et al. Use of emollients in atopic dermatitis: a questionnaire survey study. Ann Dermatol. 2014; 26:528–531.29. Lee JH, Lee SJ, Kim D, Bang D. The effect of wet-wrap dressing on epidermal barrier in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2007; 21:1360–1368.30. Boguniewicz M, Nicol N, Kelsay K, Leung DY. A multidisciplinary approach to evaluation and treatment of atopic dermatitis. Semin Cutan Med Surg. 2008; 27:115–127.31. Bindslev-Jensen C. Standardization of double-blind, placebo-controlled food challenges. Allergy. 2001; 56:Suppl 67. 75–77.32. NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010; 126:S1–S58.33. Schäfer T, Heinrich J, Wjst M, Adam H, Ring J, Wichmann HE. Association between severity of atopic eczema and degree of sensitization to aeroallergens in schoolchildren. J Allergy Clin Immunol. 1999; 104:1280–1284.34. Jeong KY, Park JW, Hong CS. House dust mite allergy in Korea: the most important inhalant allergen in current and future. Allergy Asthma Immunol Res. 2012; 4:313–325.35. Oosting AJ, de Bruin-Weller MS, Terreehorst I, Tempels-Pavlica Z, Aalberse RC, de Monchy JG, et al. Effect of mattress encasings on atopic dermatitis outcome measures in a double-blind, placebocontrolled study: the Dutch mite avoidance study. J Allergy Clin Immunol. 2002; 110:500–506.36. Sidbury R, Tom WL, Bergman JN, Cooper KD, Silverman RA, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014; 71:1218–1233.37. Fonacier LS, Aquino MR. The role of contact allergy in atopic dermatitis. Immunol Allergy Clin North Am. 2010; 30:337–350.38. Lio PA, Lee M, LeBovidge J, Timmons KG, Schneider L. Clinical management of atopic dermatitis: practical highlights and updates from the atopic dermatitis practice parameter 2012. J Allergy Clin Immunol Pract. 2014; 2:361–369.39. Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012; 26:1045–1060.40. Friedlander SF, Hebert AA, Allen DB. Fluticasone Pediatrics Safety Study Group. Safety of fluticasone propionate cream 0.05% for the treatment of severe and extensive atopic dermatitis in children as young as 3 months. J Am Acad Dermatol. 2002; 46:387–393.41. Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ. 2003; 326:1367.42. Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A, et al. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013; 1:142–151.43. Hultsch T, Kapp A, Spergel J. Immunomodulation and safety of topical calcineurin inhibitors for the treatment of atopic dermatitis. Dermatology. 2005; 211:174–187.44. Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012; 13:113–123.45. Queille-Roussel C, Paul C, Duteil L, Lefebvre MC, Rapatz G, Zagula M, et al. The new topical ascomycin derivative SDZ ASM 981 does not induce skin atrophy when applied to normal skin for 4 weeks: a randomized, double-blind controlled study. Br J Dermatol. 2001; 144:507–513.46. Paller AS, Eichenfield LF, Kirsner RS, Shull T, Jaracz E, Simpson EL, et al. Three times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for use. Pediatrics. 2008; 122:e1210–e1218.47. Hanifin JM, Paller AS, Eichenfield L, Clark RA, Korman N, Weinstein G, et al. Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis. J Am Acad Dermatol. 2005; 53:S186–S194.48. Papp KA, Werfel T, Fölster-Holst R, Ortonne JP, Potter PC, de Prost Y, et al. Long-term control of atopic dermatitis with pimecrolimus cream 1% in infants and young children: a two-year study. J Am Acad Dermatol. 2005; 52:240–246.49. Arellano FM, Wentworth CE, Arana A, Fernández C, Paul CF. Risk of lymphoma following exposure to calcineurin inhibitors and topical steroids in patients with atopic dermatitis. J Invest Dermatol. 2007; 127:808–816.50. Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011; 128:1139–1150.e4.51. Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999; 135:1522–1525.52. Simons FE. Prevention of acute urticaria in young children with atopic dermatitis. J Allergy Clin Immunol. 2001; 107:703–706.53. Ong PY, Leung DY. The infectious aspects of atopic dermatitis. Immunol Allergy Clin North Am. 2010; 30:309–321.54. Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003; 15:399–404.55. Niebuhr M, Mai U, Kapp A, Werfel T. Antibiotic treatment of cutaneous infections with Staphylococcus aureus in patients with atopic dermatitis: current antimicrobial resistances and susceptibilities. Exp Dermatol. 2008; 17:953–957.56. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009; 123:e808–e814.57. Wollenberg A, Wetzel S, Burgdorf WH, Haas J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003; 112:667–674.58. Lübbe J. Secondary infections in patients with atopic dermatitis. Am J Clin Dermatol. 2003; 4:641–654.59. Lintu P, Savolainen J, Kortekangas-Savolainen O, Kalimo K. Systemic ketoconazole is an effective treatment of atopic dermatitis with IgE-mediated hypersensitivity to yeasts. Allergy. 2001; 56:512–517.60. Mayser P, Kupfer J, Nemetz D, Schäfer U, Nilles M, Hort W, et al. Treatment of head and neck dermatitis with ciclopiroxolamine cream--results of a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2006; 19:153–158.61. Czech W, Bräutigam M, Weidinger G, Schöpf E. A body-weight-independent dosing regimen of cyclosporine microemulsion is effective in severe atopic dermatitis and improves the quality of life. J Am Acad Dermatol. 2000; 42:653–659.62. Lyakhovitsky A, Barzilai A, Heyman R, Baum S, Amichai B, Solomon M, et al. Low-dose methotrexate treatment for moderate-to-severe atopic dermatitis in adults. J Eur Acad Dermatol Venereol. 2010; 24:43–49.63. Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002; 147:324–330.64. Hanifin JM, Schneider LC, Leung DY, Ellis CN, Jaffe HS, Izu AE, et al. Recombinant interferon gamma therapy for atopic dermatitis. J Am Acad Dermatol. 1993; 28:189–197.65. Schneider LC, Baz Z, Zarcone C, Zurakowski D. Long-term therapy with recombinant interferon-gamma (rIFN-gamma) for atopic dermatitis. Ann Allergy Asthma Immunol. 1998; 80:263–268.66. Stevens SR, Hanifin JM, Hamilton T, Tofte SJ, Cooper KD. Long-term effectiveness and safety of recombinant human interferon gamma therapy for atopic dermatitis despite unchanged serum IgE levels. Arch Dermatol. 1998; 134:799–804.67. Krutmann J. Phototherapy for atopic dermatitis. Clin Exp Dermatol. 2000; 25:552–558.68. Majoie IM, Oldhoff JM, van Weelden H, Laaper-Ertmann M, Bousema MT, Sigurdsson V, et al. Narrowband ultraviolet B and medium-dose ultraviolet A1 are equally effective in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol. 2009; 60:77–84.69. Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014; 71:327–349.70. Werfel T, Breuer K, Ruff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006; 61:202–205.71. Pajno GB, Caminiti L, Vita D, Barberio G, Salzano G, Lombardo F, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2007; 120:164–170.72. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013; 132:110–117.73. Compalati E, Rogkakou A, Passalacqua G, Canonica GW. Evidences of efficacy of allergen immunotherapy in atopic dermatitis: an updated review. Curr Opin Allergy Clin Immunol. 2012; 12:427–433.74. Simon D, Hösli S, Kostylina G, Yawalkar N, Simon HU. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol. 2008; 121:122–128.75. Lane JE, Cheyney JM, Lane TN, Kent DE, Cohen DJ. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006; 54:68–72.76. Belloni B, Ziai M, Lim A, Lemercier B, Sbornik M, Weidinger S, et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. J Allergy Clin Immunol. 2007; 120:1223–1225.77. Krathen RA, Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. J Am Acad Dermatol. 2005; 53:338–340.78. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007; 370:1422–1431.79. Beck LA, Thai D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014; 371:130–139.80. Jacobi A, Antoni C, Manger B, Schuler G, Hertl M. Infliximab in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol. 2005; 52:522–526.81. Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005; 60:693–696.82. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014; 371:1189–1197.83. Navarini AA, French LE, Hofbauer GF. Interrupting IL-6-receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J Allergy Clin Immunol. 2011; 128:1128–1130.84. Raap U, Weißmantel S, Gehring M, Eisenberg AM, Kapp A, Fölster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol. 2012; 23:285–288.85. Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014; 370:2102–2110.86. Bamford JT, Ray S, Musekiwa A, van Gool C, Humphreys R, Ernst E. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013; 4:CD004416.87. Koch C, Dölle S, Metzger M, Rasche C, Jungclas H, Rühl R, et al. Docosahexaenoic acid (DHA) supplementation in atopic eczema: a randomized, double-blind, controlled trial. Br J Dermatol. 2008; 158:786–792.88. Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997; 159:1739–1745.89. Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014; 71:814–821.90. Bath-Hextall FJ, Jenkinson C, Humphreys R, Williams HC. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012; 2:CD005205.91. Hughes R, Ward D, Tobin AM, Keegan K, Kirby B. The use of alternative medicine in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2007; 24:118–120.92. Lee JH, Cho SH. Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 2011; 133:810–817.93. Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol. 2011; 164:1078–1082.94. Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013; 131:295–299.e27 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severe Eczema Herpeticum in an Adult Patient with Atopic Dermatitis Following Herbal Medicine and Acupuncture
- A Case of Eczema Herpeticum Occurring in Atopic Dermatitis
- A Case of Dirty Neck' of Atopics
- Comparison of Clinical Severity and Laboratory Results between Atopic and Non-atopic Eczema in Children
- A Case of Unilateral Nipple Eczema Developing after Chronic Scratch in Atopic Dermatitis Patient