J Korean Med Sci.
2017 Nov;32(11):1857-1860. 10.3346/jkms.2017.32.11.1857.
Clevudine Induced Mitochondrial Myopathy
- Affiliations
-
- 1Department of Neurology, Dongguk University Ilsan Hospital, Goyang, Korea. nheekim8@hanmail.net
- 2Department of Neurology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Neurology, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
- 4Department of Hepatology, Dongguk University Ilsan Hospital, Goyang, Korea.
- KMID: 2390315
- DOI: http://doi.org/10.3346/jkms.2017.32.11.1857
Abstract
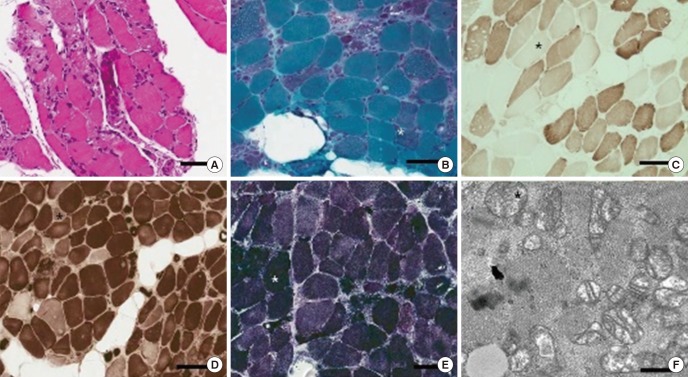
- Clevudine was approved as an antiviral agent for hepatitis B virus, which showed marked, rapid inhibition of virus replication without significant toxicity. However, several studies have reported myopathy associated with clevudine therapy. Also, we experienced seven patients who suffered from myopathy during clevudine therapy. To characterize clevudine-induced myopathy, we collected previously reported cases of clevudine myopathy and analyzed all the cases including our cases. We searched electronic databases that were published in English or Korean using PubMed and KoreaMed. Ninety-five cases with clevudine myopathy, including our seven cases, were selected and analyzed for the demographic data, clinical features, and pathologic findings. The 95 patients with clevudine-induced myopathy comprised 52 women and 43 men aged 48.9 years (27-76 years). The patients received clevudine therapy for about 14.2 months (5-24 months) before the development of symptoms. Weakness mainly involved proximal extremities, especially in the lower extremities, and bulbar and neck weakness were observed in some cases (13.7%). Creatine kinase was elevated in the majority of patients (97.9%). Myopathic patterns on electromyography were observed in most patients examined (98.1%). Muscle biopsy presented patterns compatible with mitochondrial myopathy in the majority (90.2%). The weakness usually improved within about 3 months after the discontinuation of clevudine. Though clevudine has been known to be safe in a 6-month clinical trial, longer clevudine therapy for about 14 months may cause reversible mitochondrial myopathy. Careful clinical attention should be paid to patients with long-term clevudine therapy.
MeSH Terms
Figure
Reference
-
1. Lam YF, Yuen MF, Seto WK, Lai CL. Current antiviral therapy of chronic hepatitis B: efficacy and safety. Curr Hepat Rep. 2011; 10:235–243. PMID: 22131901.2. Lok AS, McMahon BJ, Brown RS Jr, Wong JB, Ahmed AT, Farah W, Almasri J, Alahdab F, Benkhadra K, Mouchli MA, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016; 63:284–306. PMID: 26566246.3. Korba BE, Furman PA, Otto MJ. Clevudine: a potent inhibitor of hepatitis B virus in vitro and in vivo. Expert Rev Anti Infect Ther. 2006; 4:549–561. PMID: 17009935.4. Yoo BC, Kim JH, Chung YH, Lee KS, Paik SW, Ryu SH, Han BH, Han JY, Byun KS, Cho M, et al. Twenty-four-week clevudine therapy showed potent and sustained antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology. 2007; 45:1172–1178. PMID: 17464992.5. Manzoor S, Saalim M, Imran M, Resham S, Ashraf J. Hepatitis B virus therapy: what's the future holding for us? World J Gastroenterol. 2015; 21:12558–12575. PMID: 26640332.6. Seok JI, Lee DK, Lee CH, Park MS, Kim SY, Kim HS, Jo HY, Lee CH, Kim DS. Long-term therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA. Hepatology. 2009; 49:2080–2086. PMID: 19333909.7. Kim BK, Oh J, Kwon SY, Choe WH, Ko SY, Rhee KH, Seo TH, Lim SD, Lee CH. Clevudine myopathy in patients with chronic hepatitis B. J Hepatol. 2009; 51:829–834. PMID: 19615776.8. Kim JY, Yoon YS, Park KD, Koo H. Myopathy due to chronic clevudine therapy: a case report. Korean J Pathol. 2009; 43:575–579.9. Yang CY, Park SA, Kim HS, Shin YI. Polymyositis in patients taking antiviral clevudine therapy: a report of two cases. NeuroRehabilitation. 2010; 26:159–162. PMID: 20203382.10. Seok JI. Clinical and pathological features of clevudine induced myopathy. J Korean Neurol Assoc. 2013; 31:26–31.11. Lee JW, Lee YJ, Lee JJ, Kim JH, Jung YK, Kwon OS, Choi DJ, Kim YS, Kim JH. Efficacy of entecavir switching therapy in chronic hepatitis B patients with clevudine-induced myopathy. Korean J Gastroenterol. 2013; 61:30–36. PMID: 23354347.12. Tak WY, Park SY, Cho CM, Jung MK, Jeon SW, Kweon YO, Park JY, Sohn YK. Clinical, biochemical, and pathological characteristics of clevudine-associated myopathy. J Hepatol. 2010; 53:261–266. PMID: 20466447.13. Jang JH, Kim JW, Jeong SH, Myung HJ, Kim HS, Park YS, Lee SH, Hwang JH, Kim N, Lee DH. Clevudine for chronic hepatitis B: antiviral response, predictors of response, and development of myopathy. J Viral Hepat. 2011; 18:84–90. PMID: 20196804.14. Yoon EL, Yim HJ, Lee HJ, Lee YS, Kim JH, Jung ES, Kim JH, Seo YS, Yeon JE, Lee HS, et al. Comparison of clevudine and entecavir for treatment-naive patients with chronic hepatitis B virus infection: two-year follow-up data. J Clin Gastroenterol. 2011; 45:893–899. PMID: 21617542.15. Scruggs ER, Dirks Naylor AJ. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008; 82:83–88. PMID: 18504416.16. Masini A, Scotti C, Calligaro A, Cazzalini O, Stivala LA, Bianchi L, Giovannini F, Ceccarelli D, Muscatello U, Tomasi A, et al. Zidovudine-induced experimental myopathy: dual mechanism of mitochondrial damage. J Neurol Sci. 1999; 166:131–140. PMID: 10475107.17. Ambang T, Tan JS, Ong S, Wong KT, Goh KJ. Clinicopathological features of Telbivudine-associated myopathy. PLoS One. 2016; 11:e0162760. PMID: 27611456.18. Gwak GY, Eo SJ, Shin SR, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. A comparison of clevudine and entecavir for treatment-naïve patients with chronic hepatitis B: results after 2 years of treatment. Hepatol Int. 2013; 7:106–110. PMID: 26201625.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical and Pathological Features of Clevudine Induced Myopathy
- Efficacy of Entecavir Switching Therapy in Chronic Hepatitis B Patients with Clevudine-induced Myopathy
- Myopathy due to Chronic Clevudine Therapy: A Case Report
- Enzyme histochemical study of germanium dioxide-induced mitochondrial myopathy in rats
- Clevudine therapy in patients with chronic hepatitis B