Ann Rehabil Med.
2017 Jun;41(3):456-464. 10.5535/arm.2017.41.3.456.
Cardiopulmonary Exercise Test in Leukemia Patients After Chemotherapy: A Feasibility Study
- Affiliations
-
- 1Department of Rehabilitation Medicine, Chungnam National University Hospital, Daejeon, Korea. drjeesungju@hanmail.net
- 2Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Korea.
- KMID: 2389462
- DOI: http://doi.org/10.5535/arm.2017.41.3.456
Abstract
OBJECTIVE
To explore the feasibility of cardiopulmonary exercise test (CPET) in leukemia patients after chemotherapy.
METHODS
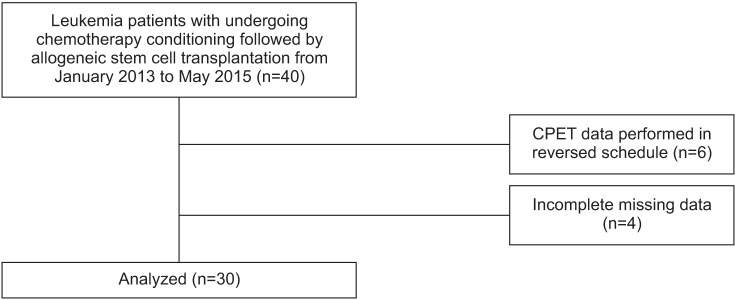
Leukemia patients with histologically confirmed hematologic malignancies were reviewed. We evaluated for CPET, between receiving chemotherapy and undergoing stem cell transplantation after 2 weeks. We recorded exercise testing and physiologic parameters during CPET between January 2013 to May 2015. All patients were subjected to symptoms limited to exercise testing, according to the Modified Bruce Protocol. We considered that if respiratory exchange ratio achieved was over 1.10, participants had successfully completed CPET. We dichotomized all participants into two groups (normal group, normal range of resting heart rate; higher group, over 100 per minute of heart rate).
RESULTS
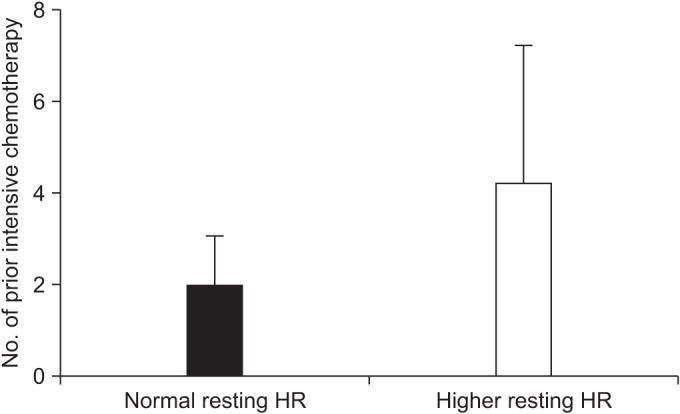
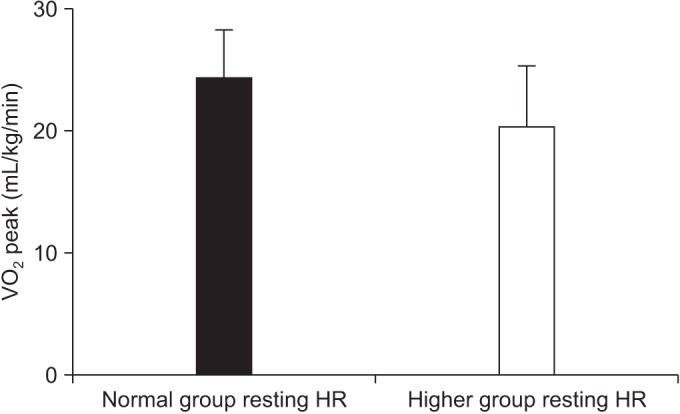
30 patients were finally enrolled. All participants had no adverse effects during the exercise test. Mean peak double product was 26,998.60 mmHg·beats/min (range, 15,481-41,004), and mean peak oxygen consumption (VOâ‚‚ peak) was 22.52±4.56 mL/kg/min. Significant differences were observed in the normal group with VOâ‚‚ peak (mean, 24.21 mL/kg/min; p=0.027) and number of prior intensive chemotherapy, compared to the higher group (mean, 1.95; p=0.006).
CONCLUSION
Our results indicate that CPET in leukemia patients before stem cell transplantation was very safe, and is an efficient method to screen for patients with poor cardiac functions. As CPET presents the parameters which reveal the cardiopulmonary functions, including VOâ‚‚ peak, double product and exercise capacity, this exercise test would help to predict the physical performance or general condition of the leukemia patients.
MeSH Terms
Figure
Reference
-
1. Doocey RT, Toze CL, Connors JM, Nevill TJ, Gascoyne RD, Barnett MJ, et al. Allogeneic haematopoietic stem-cell transplantation for relapsed and refractory aggressive histology non-Hodgkin lymphoma. Br J Haematol. 2005; 131:223–230. PMID: 16197454.
Article2. Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009; 113:4489–4496. PMID: 19244158.
Article3. Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008; 111:446–452. PMID: 17916744.
Article4. Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008; 26:455–462. PMID: 18086796.
Article5. Devergie A, Blaise D, Attal M, Tigaud JD, Jouet JP, Vernant JP, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM). Blood. 1995; 85:2263–2268. PMID: 7718899.
Article6. Hartman AR, Williams SF, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant. 1998; 22:439–443. PMID: 9733266.7. Kroger N, Zabelina T, Kruger W, Renges H, Stute N, Kabisch H, et al. Comparison of total body irradiation vs busulfan in combination with cyclophosphamide as conditioning for unrelated stem cell transplantation in CML patients. Bone Marrow Transplant. 2001; 27:349–354. PMID: 11313663.
Article8. Ringden O, Remberger M, Ruutu T, Nikoskelainen J, Volin L, Vindelov L, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999; 93:2196–2201. PMID: 10090927.9. Kelsey CR, Horwitz ME, Chino JP, Craciunescu O, Steffey B, Folz RJ, et al. Severe pulmonary toxicity after myeloablative conditioning using total body irradiation: an assessment of risk factors. Int J Radiat Oncol Biol Phys. 2011; 81:812–818. PMID: 20932682.
Article10. Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012; 30:2530–2537. PMID: 22614980.
Article11. Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012; 76:248–252. PMID: 22112290.
Article12. Jones LW, Watson D, Herndon JE 2nd, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010; 116:4825–4832. PMID: 20597134.
Article13. Ruden E, Reardon DA, Coan AD, Herndon JE 2nd, Hornsby WE, West M, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011; 29:2918–2923. PMID: 21690470.
Article14. Acanfora D, De Caprio L, Cuomo S, Papa M, Ferrara N, Leosco D, et al. Diagnostic value of the ratio of recovery systolic blood pressure to peak exercise systolic blood pressure for the detection of coronary artery disease. Circulation. 1988; 77:1306–1310. PMID: 3370771.
Article15. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002; 346:793–801. PMID: 11893790.
Article16. Irving JB, Bruce RA, DeRouen TA. Variations in and significance of systolic pressure during maximal exercise (treadmill) testing. Am J Cardiol. 1977; 39:841–848. PMID: 871110.
Article17. Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol. 1972; 32:516–522. PMID: 5026501.
Article18. Nelson RR, Gobel FL, Jorgensen CR, Wang K, Wang Y, Taylor HL. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation. 1974; 50:1179–1189. PMID: 4430113.
Article19. Kelsey CR, Scott JM, Lane A, Schwitzer E, West MJ, Thomas S, et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone Marrow Transplant. 2014; 49:1330–1336. PMID: 25068429.
Article20. Scardovi AB, Coletta C, De Maria R, Perna S, Aspromonte N, Feola M, et al. The cardiopulmonary exercise test is safe and reliable in elderly patients with chronic heart failure. J Cardiovasc Med (Hagerstown). 2007; 8:608–612. PMID: 17667032.
Article21. American Thoracic Society. American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003; 167:211–277. PMID: 12524257.22. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985). 1986; 60:2020–2027. PMID: 3087938.
Article23. Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs sedentary women: a meta-analysis. J Appl Physiol (1985). 1997; 83:160–165. PMID: 9216959.24. Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. Am J Physiol Heart Circ Physiol. 2000; 278:H829–H834. PMID: 10710351.25. Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007; 14:215–221. PMID: 17446799.
Article26. Tuchman SA, Lane A, Hornsby WE, Bishop C, Thomas S, Herndon JE 2nd, et al. Quantitative measures of physical functioning after autologous hematopoietic stem cell transplantation in multiple myeloma: a feasibility study. Clin Lymphoma Myeloma Leuk. 2015; 15:103–109. PMID: 25445473.
Article27. Sadrzadeh Rafie AH, Sungar GW, Dewey FE, Hadley D, Myers J, Froelicher VF. Prognostic value of double product reserve. Eur J Cardiovasc Prev Rehabil. 2008; 15:541–547. PMID: 18665099.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Cardiopulmonary Function in the Patients with Ankylosing Spondylitis Using Exercise Stress Test
- Early Response of Cardiopulmonary Exercise Test in Patients with Locally Advanced Non-Small Cell Lung Cancer Treated with Systemic Chemotherapy
- Early response of cardiopulmonary exercise test(CPET) in patients with locally advanced Non-Small Cell Lung cancer treated with radiation
- Cardiopulmonary Exercise Testing and Interpretation of the Major Related Variables in Patients with Congenital Heart Disease
- Breathing Reserve Index at Anaerobic Threshold of Cardiopulmonary Exercise Test in Chronic Obstructive Pulmonary Disease