Korean J Orthod.
2017 Sep;47(5):306-312. 10.4041/kjod.2017.47.5.306.
The effect of fluoride-containing oral rinses on the corrosion resistance of titanium alloy (Ti-6Al-4V)
- Affiliations
-
- 1Department of Orthodontics, College of Dentistry, Yonsei University, Seoul, Korea.
- 2Department and Research Institute of Dental Biomaterials and Bioengineering, College of Dentistry, Yonsei University, Seoul, Korea. kmkim@yuhs.ac
- 3Department of Orthodontics, The Institute of Craniofacial Deformity, College of Dentistry, Yonsei University, Seoul, Korea. hwang@yuhs.ac
- 4BK21 PLUS Project, Yonsei University College of Dentistry.
- KMID: 2389146
- DOI: http://doi.org/10.4041/kjod.2017.47.5.306
Abstract
OBJECTIVE
The purpose of this study was to examine the effect of commercially available fluoride-containing oral rinses on the corrosion behavior of titanium alloys, which are the main components of orthodontic miniscrews.
METHODS
Four commercially available oral rinses (solution A, pH 4.46/260 ppm fluoride; solution B, pH 4.41/178 ppm fluoride; solution C, pH 6.30/117 ppm fluoride; and solution D, pH 4.17/3.92 ppm fluoride) were tested on titanium alloy (Ti-6Al-4V) circular plates, and saline was used as the control. The open-circuit potential and potentiodynamic polarization of these materials were measured. Thereafter, all samples were evaluated under a field-emission scanning electron microscope.
RESULTS
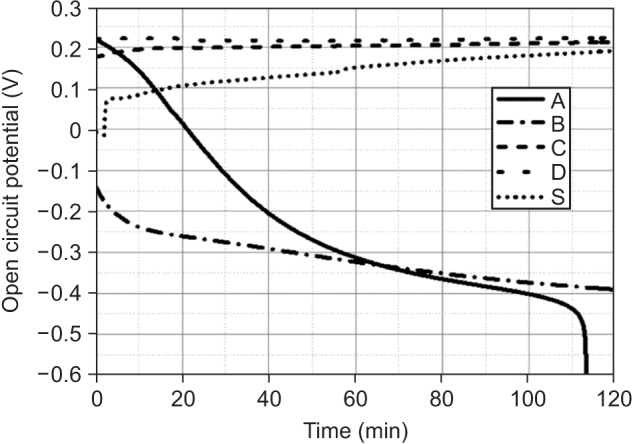
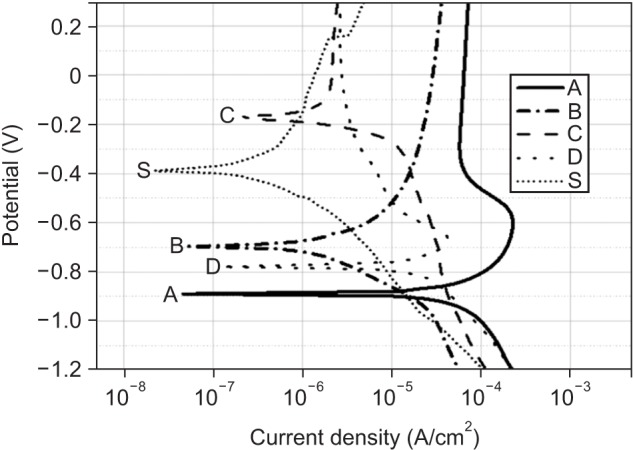
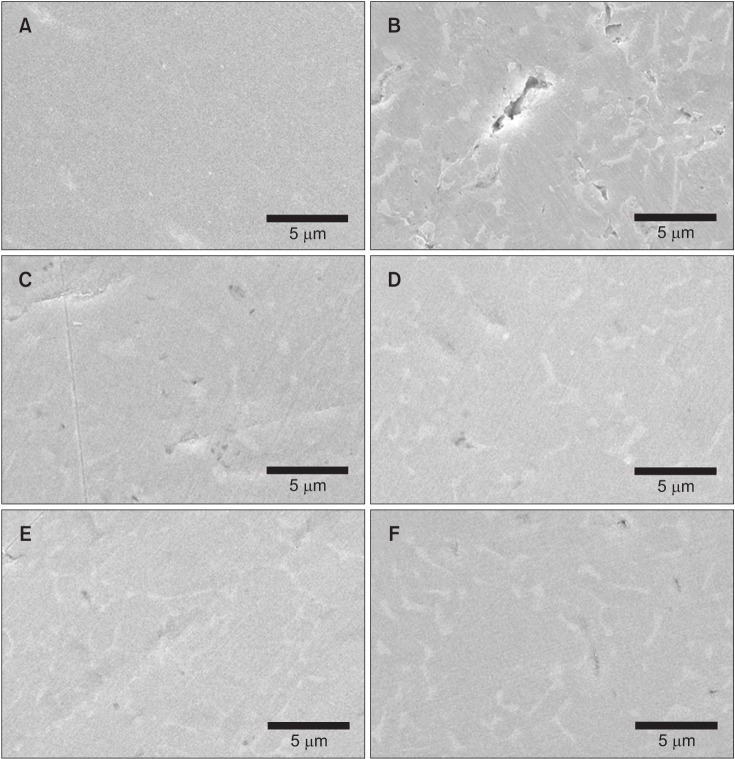
Among the tested oral rinses, except solution D, the more the fluoride content was, the greater was the corrosion potential downtrend; the corrosion resistance of the titanium alloy sample was also lowered significantly (p < 0.05). Field-emission scanning electron microscopic analysis of the surface morphology of the titanium alloy samples revealed that all samples had some defects, crevices, or pitting after exposure to the oral rinses than before treatment. In particular, the samples in solution A showed the most changes.
CONCLUSIONS
Commercially available oral rinses having a high fluoride concentration and a low pH may reduce the corrosion resistance of titanium alloys used in dental appliances such as orthodontic titanium miniscrews and brackets.
Keyword
Figure
Reference
-
1. Choi SH, Jeong WS, Cha JY, Lee JH, Yu HS, Choi EH, et al. Time-dependent effects of ultraviolet and nonthermal atmospheric pressure plasma on the biological activity of titanium. Sci Rep. 2016; 6:33421. PMID: 27627871.
Article2. Choi SH, Kim SJ, Lee KJ, Sung SJ, Chun YS, Hwang CJ. Stress distributions in peri-miniscrew areas from cylindrical and tapered miniscrews inserted at different angles. Korean J Orthod. 2016; 46:189–198. PMID: 27478796.
Article3. Park HS, Jeong SH, Kwon OW. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2006; 130:18–25. PMID: 16849067.
Article4. Cheng SJ, Tseng IY, Lee JJ, Kok SH. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants. 2004; 19:100–106. PMID: 14982362.5. Venugopal A, Muthuchamy N, Tejani H, Gopalan AI, Lee KP, Lee HJ, et al. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J Orthod. 2017; 47:3–10. PMID: 28127534.
Article6. Sudjalim TR, Woods MG, Manton DJ, Reynolds EC. Prevention of demineralization around orthodontic brackets in vitro. Am J Orthod Dentofacial Orthop. 2007; 131:705.e1–705.e9.
Article7. Mellberg JR, Ripa LW. Fluoride in preventive dentistry: theory and clinical applications. Chicago: Quintessence Pub Co;1983.8. Lopatiene K, Borisovaite M, Lapenaite E. Prevention and treatment of white spot lesions during and after treatment with fixed orthodontic appliances: a systematic literature review. J Oral Maxillofac Res. 2016; 7:e1.
Article9. Toumelin-Chemla F, Rouelle F, Burdairon G. Corrosive properties of fluoride-containing odontologic gels against titanium. J Dent. 1996; 24:109–115. PMID: 8636481.
Article10. Lausmaa J, Kasemo B, Hansson S. Accelerated oxide growth on titanium implants during autoclaving caused by fluorine contamination. Biomaterials. 1985; 6:23–27. PMID: 3971014.
Article11. Könönen MH, Lavonius ET, Kivilahti JK. SEM observations on stress corrosion cracking of commercially pure titanium in a topical fluoride solution. Dent Mater. 1995; 11:269–272. PMID: 8621050.
Article12. Boere G. Influence of fluoride on titanium in an acidic environment measured by polarization resistance technique. J Appl Biomater. 1995; 6:283–288. PMID: 8589513.
Article13. Souza JCM, Barbosa SL, Ariza E, Celis JP, Rocha LA. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear. 2012; 292-293:82–88.
Article14. Oshida Y, Sellers CB, Mirza K, Farzin-Nia F. Corrosion of dental metallic materials by dental treatment agents. Mater Sci Eng C. 2005; 25:343–348.
Article15. Nakagawa M, Matsuya S, Shiraishi T, Ohta M. Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J Dent Res. 1999; 78:1568–1572. PMID: 10512392.
Article16. Wang JJ, Sanderson BJ, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007; 628:99–106. PMID: 17223607.
Article17. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000; 82:457–476. PMID: 10761937.
Article18. Manda MG, Psyllaki PP, Tsipas DN, Koidis PT. Observations on an in-vivo failure of a titanium dental implant/abutment screw system: a case report. J Biomed Mater Res B Appl Biomater. 2009; 89:264–273. PMID: 18837452.19. Manaranche C, Hornberger H. A proposal for the classification of dental alloys according to their resistance to corrosion. Dent Mater. 2007; 23:1428–1437. PMID: 17466365.
Article20. Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007; 28:5044–5048. PMID: 17645943.
Article21. Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, et al. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br. 1994; 76:701–712. PMID: 8083255.
Article22. Broggini N, McManus LM, Hermann JS, Medina RU, Oates TW, Schenk RK, et al. Persistent acute inflammation at the implant-abutment interface. J Dent Res. 2003; 82:232–237. PMID: 12598555.
Article23. Guindy JS, Schiel H, Schmidli F, Wirz J. Corrosion at the marginal gap of implant-supported suprastructures and implant failure. Int J Oral Maxillofac Implants. 2004; 19:826–831. PMID: 15623057.24. Oh EJ, Nguyen TD, Lee SY, Jeon YM, Bae TS, Kim JG. Enhanced compatibility and initial stability of Ti6Al4V alloy orthodontic miniscrews subjected to anodization, cyclic precalcification, and heat treatment. Korean J Orthod. 2014; 44:246–253. PMID: 25309864.
Article25. Golvano I, Garcia I, Conde A, Tato W, Aginagalde A. Influence of fluoride content and pH on corrosion and tribocorrosion behaviour of Ti13Nb13Zr alloy in oral environment. J Mech Behav Biomed Mater. 2015; 49:186–196. PMID: 26042765.
Article26. Muguruma T, Iijima M, Brantley WA, Yuasa T, Kyung HM, Mizoguchi I. Effects of sodium fluoride mouth rinses on the torsional properties of miniscrew implants. Am J Orthod Dentofacial Orthop. 2011; 139:588–593. PMID: 21536200.
Article27. Souza JC, Barbosa SL, Ariza EA, Henriques M, Teughels W, Ponthiaux P, et al. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater Sci Eng C Mater Biol Appl. 2015; 47:384–393. PMID: 25492211.
Article28. Nakagawa M, Matsuya S, Udoh K. Corrosion behavior of pure titanium and titanium alloys in fluoride-containing solutions. Dent Mater J. 2001; 20:305–314. PMID: 11915624.
Article29. Blackwood DJ, Peter LM, Williams DE. Stability and open circuit breakdown of the passive oxide film on titanium. Electroch Acta. 1988; 33:1143–1149.
Article30. Robin A, Meirelis JP. Influence of fluoride concentration and pH on corrosion behavior of titanium in artificial saliva. J Appl Electroch. 2007; 37:511–517.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the resistance of wear and cytotoxicity of the titanium surface after film depositions
- Osteoblastic behavior to zirconium coating on Ti-6Al-4V alloy
- A HISTOMORPHOMETRIC STUDY OF BONE APPOSITION TO NEWLY DEVELOPED TI-BASED ALLOYS IN RABBIT BONE
- Comparison of Cytocompatibility Between Grit Blasted Titanium Alloy (Ti-6Al-4V) with or without Pure Titanium Coating
- The evaluation of cytotoxicity and biocompatibility of Ti-Ta-Nb-base alloy