Korean J Physiol Pharmacol.
2011 Feb;15(1):17-22.
Quercetin Inhibits alpha3beta4 Nicotinic Acetylcholine Receptor-Mediated Ion Currents Expressed in Xenopus Oocytes
- Affiliations
-
- 1Department of Physiology, College of Veterinary Medicine and Bio/Molecular Informatics Center, Konkuk University, Seoul 143-701, Korea. synah@konkuk.ac.kr
Abstract
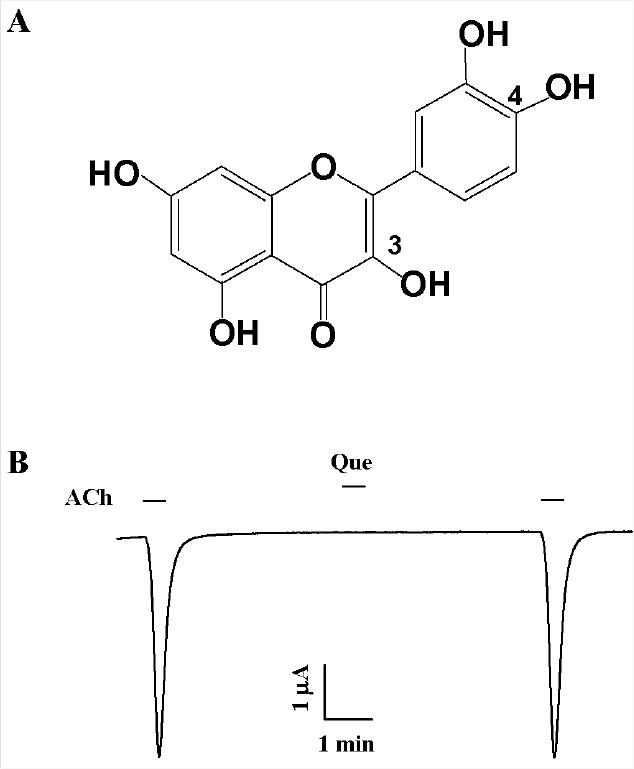
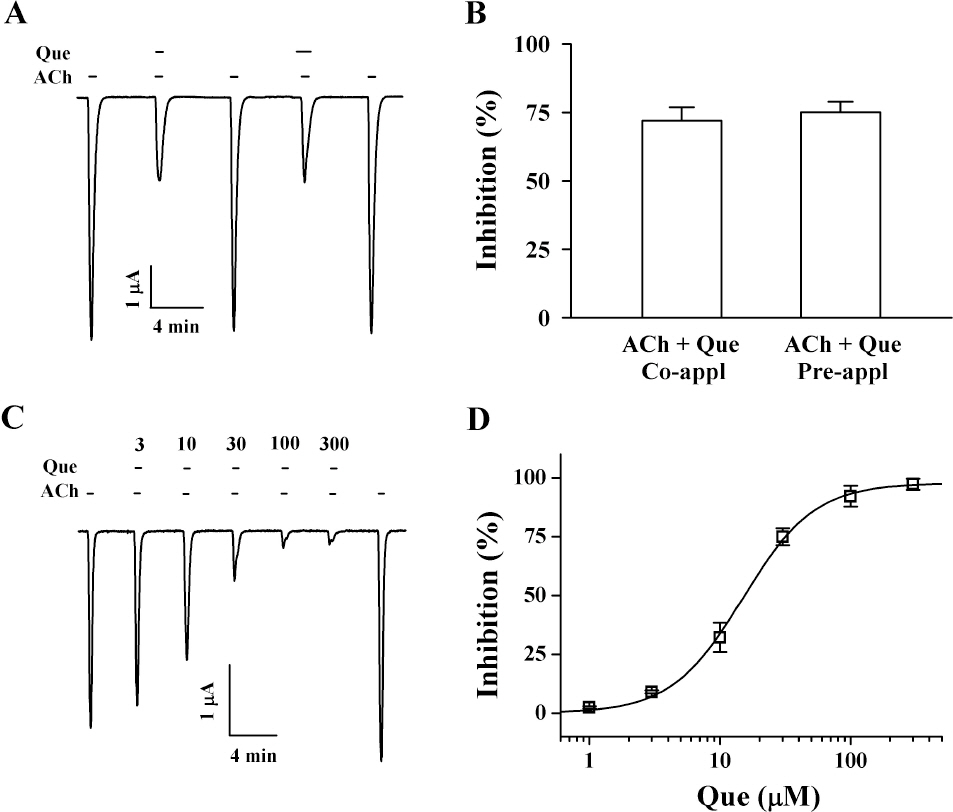
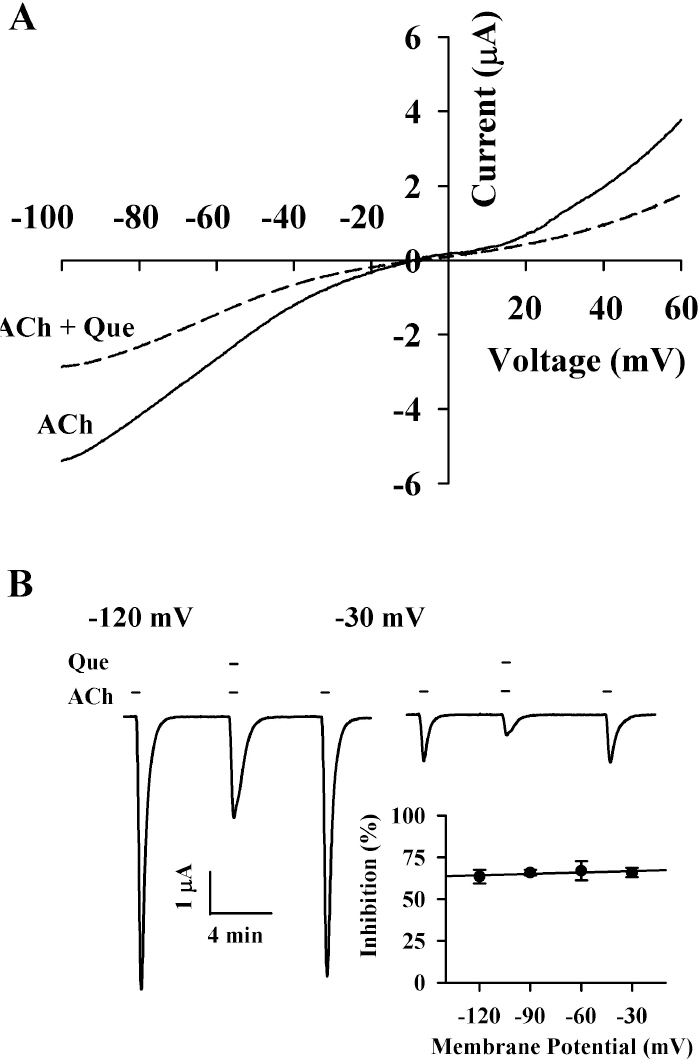
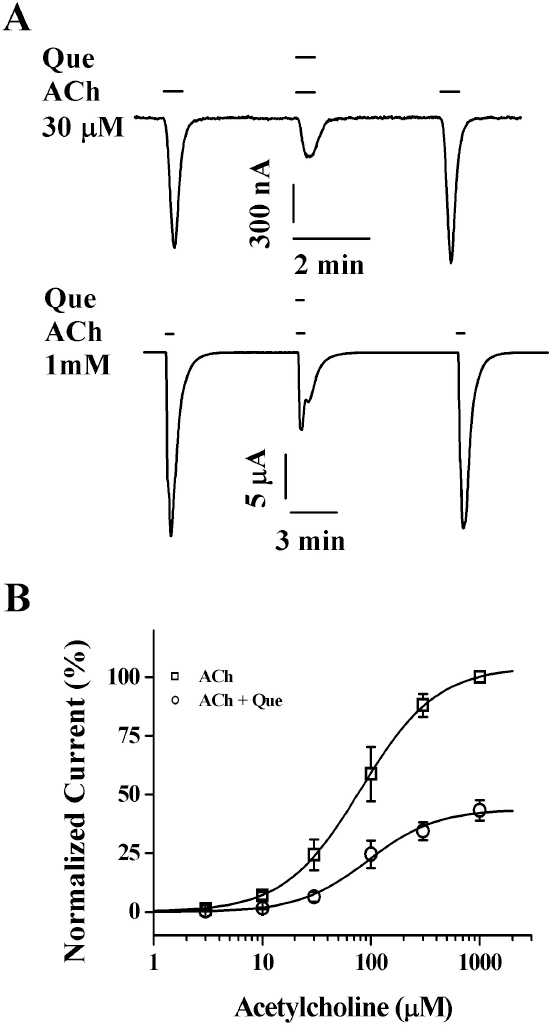
- Quercetin mainly exists in the skin of colored fruits and vegetables as one of flavonoids. Recent studies show that quercetin, like other flavonoids, has diverse pharmacological actions. However, relatively little is known about quercetin effects in the regulations of ligand-gated ion channels. In the previous reports, we have shown that quercetin regulates subsets of homomeric ligand-gated ion channels such as glycine, 5-HT3A and alpha7 nicotinic acetylcholine receptors. In the present study, we examined quercetin effects on heteromeric neuronal alpha3beta4 nicotinic acetylcholine receptor channel activity expressed in Xenopus oocytes after injection of cRNA encoding bovine neuronal alpha3 and beta4 subunits. Treatment with acetylcholine elicited an inward peak current (IACh) in oocytes expressing alpha3beta4 nicotinic acetylcholine receptor. Co-treatment with quercetin and acetylcholine inhibited IACh in oocytes expressing alpha3beta4 nicotinic acetylcholine receptors. The inhibition of IACh by quercetin was reversible and concentration-dependent. The half-inhibitory concentration (IC50) of quercetin was 14.9+/-0.8 microM in oocytes expressing alpha3beta4 nicotinic acetylcholine receptor. The inhibition of IACh by quercetin was voltage-independent and non-competitive. These results indicate that quercetin might regulate alpha3beta4 nicotinic acetylcholine receptor and this regulation might be one of the pharmacological actions of quercetin in nervous systems.
MeSH Terms
Figure
Reference
-
References
1. Jensen ML, Schousboe A, Ahring PK. Charge selectivity of the Cys-loop family of ligand-gated ion channels. J Neurochem. 2005; 92:217–225.
Article2. Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007; 47:699–729.
Article3. Boulter J, Evans K, Goldman D, Martin G, Treco D, Heinemann S, Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. Nature. 1986; 319:368–374.4. Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987; 84:7763–7767.
Article5. Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002; 3:102–114.
Article6. Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004; 74:363–396.
Article7. Boorman JP, Groot-Kormelink PJ, Sivilotti LG. Stoichiometry of human recombinant neuronal nicotinic receptors containing the b3 subunit expressed in Xenopus oocytes. J Physiol. 2000; 529:565–577.8. Free RB, McKay SB, Boyd RT, McKay DB. Evidence for constitutive expression of bovine adrenal a3beta4∗ nicotinic acetylcholine receptors. Ann N Y Acad Sci. 2002; 971:145–147.9. Di Angelantonio S, Matteoni C, Fabbretti E, Nistri A. Molecular biology and electrophysiology of neuronal nicotinic receptors of rat chromaffin cells. Eur J Neurosci. 2003; 17:2313–2322.
Article10. Campos-Caro A, Smillie FI, Domínguez del Toro E, Rovira JC, Vicente-Agulló F, Chapuli J, Juíz JM, Sala S, Sala F, Ballesta JJ, Criado M. Neuronal nicotinic acetylcholine receptors on bovine chromaffin cells: cloning, expression, and genomic organization of receptor subunits. J Neurochem. 1997; 68:488–497.
Article11. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002; 96:67–202.
Article12. Speroni E, Minghetti A. Neuropharmacological activity of extracts from Passiflora incarnata. Planta Med. 1988; 54:488–491.13. Picq M, Cheav SL, Prigent AF. Effect of two flavonoid compounds on central nervous system. Analgesic activity. Life Sci. 1991; 49:1979–1988.
Article14. Kandaswami C, Middleton E Jr. Free radical scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol. 1994; 366:351–376.
Article15. Oyama Y, Fuchs PA, Katayama N, Noda K. Myricetin and quercetin, the flavonoid constituents of Ginkgo biloba extract, greatly reduce oxidative metabolism in both resting and Ca(2+)-loaded brain neurons. Brain Res. 1994; 635:125–129.16. Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000; 55:481–504.
Article17. Lee BH, Jeong SM, Lee JH, Kim JH, Yoon IS, Lee JH, Choi SH, Lee SM, Chang CG, Kim HC, Han Y, Paik HD, Kim Y, Nah SY. Quercetin inhibits the 5-hydroxytryptamine type 3 receptor-mediated ion current by interacting with pre-transmembrane domain I. Mol Cells. 2005; 20:69–73.18. Lee BH, Lee JH, Yoon IS, Lee JH, Choi SH, Pyo MK, Jeong SM, Choi WS, Shin TJ, Lee SM, Rhim H, Park YS, Han YS, Paik HD, Cho SG, Kim CH, Lim YH, Nah SY. Human glycine alpha1 receptor inhibition by quercetin is abolished or inversed by alpha267 mutations in transmembrane domain 2. Brain Res. 2007; 1161:1–10.19. Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Kim BR, Lee SM, Lee JH, Kim HC, Park HY, Rhim H, Nah SY. Quercetin enhances human α7 nicotinic acetylcholine receptor-mediated ion current through interactions with Ca2+ binding sites. Mol Cells. 2010; 30:245–253.20. Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Lee SM, Paik HD, Kim HC, Nah SY. Effects of quercetin on α9α10 nicotinic acetylcholine receptor-mediated ion currents. Eur J Pharmacol. 2011; 650:79–85.21. Sine SM, Taylor P. Local anesthetics and histrionicotoxin are allosteric inhibitors of the acetylcholine receptor. Studies of clonal muscle cells. J Biol Chem. 1982; 257:8106–8114.
Article22. Heidmann T, Oswald RE, Changeux JP. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983; 22:3112–3127.
Article23. Arias HR. Luminal and non-luminal non-competitive inhibitor binding sites on the nicotinic acetylcholine receptor. Mol Membr Biol. 1996; 13:1–17.
Article24. Gr⊘nlien JH, Ween H, Thorin-Hagene K, Cassar S, Li J, Briggs CA, Gopalakrishnan M, Malysz J. Importance of M2-M3 loop in governing properties of genistein at the α7 nicotinic acetylcholine receptor inferred from α7/5-HT3A chimera. Eur J Pharmacol. 2010; 647:37–47.25. Zhang H, Toyohira Y, Ueno S, Shinohara Y, Itoh H, Furuno Y, Yamakuni T, Tsutsui M, Takahashi K, Yanagihara N. Dual effects of nobiletin, a citrus polymethoxy flavone, on catecholamine secretion in cultured bovine adrenal medullary cells. J Neurochem. 2010; 114:1030–1038.
Article26. Shinohara Y, Toyohira Y, Ueno S, Liu M, Tsutsui M, Yanagihara N. Effects of resveratrol, a grape polyphenol, on catecholamine secretion and synthesis in cultured bovine adrenal medullary cells. Biochem Pharmacol. 2007; 74:1608–1618.
Article27. Yu BS, Na DM, Kang MY, Lim DY. Polyphenols of Rubus coreanum inhibit catecholamine secretion from the perfused adrenal medulla of SHRs. Korean J Physiol Pharmacol. 2009; 13:517–526.
Article28. García-Colunga J, Miledi R. Effects of serotonergic agents on neuronal nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A. 1995; 92:2919–2923.29. García-Colunga J, Miledi R. Modulation of nicotinic acetylcholine receptors by strychnine. Proc Natl Acad Sci U S A. 1999; 96:4113–4118.30. Herrero CJ, García-Palomero E, Pintado AJ, García AG, Montiel C. Differential blockade of rat alpha3beta4 and alpha7 neuronal nicotinic receptors by omega-conotoxin MVIIC, omega-conotoxin GVIA and diltiazem. Br J Pharmacol. 1999; 127:1375–1387.31. Haghighi AP, Cooper E. A molecular link between inward rectification and calcium permeability of neuronal nicotinic acetylcholine alpha3beta4 and alpha4beta2 receptors. J Neurosci. 2000; 20:529–541.32. Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992; 89:9949–9953.
Article33. Kindler CH, Verotta D, Gray AT, Gropper MA, Yost CS. Additive inhibition of nicotinic acetylcholine receptors by corticosteroids and the neuromuscular blocking drug vecuronium. Anesthesiology. 2000; 92:821–832.
Article34. Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999; 289:774–780.35. Palma E, Maggi L, Miledi R, Eusebi F. Effects of Zn2+ on wild and mutant neuronal alpha7 nicotinic receptors. Proc Natl Acad Sci U S A. 1998; 95:10246–10250.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory Effects of Quercetin on Muscle-type of Nicotinic Acetylcholine Receptor-Mediated Ion Currents Expressed in Xenopus Oocytes
- Differential Effects of Quercetin and Quercetin Glycosides on Human α7 Nicotinic Acetylcholine Receptor-Mediated Ion Currents
- Quantitative Structure Activity Relationship between Diazabicyclo[4.2.0]octanes Derivatives and Nicotinic Acetylcholine Receptor Agonists
- Resveratrol Inhibits GABAC rho Receptor-Mediated Ion Currents Expressed in Xenopus Oocytes
- Block of ATP-sensitive K+ channels expressed in Xenopus oocytes by dimethyl sulfoxide