Ann Dermatol.
2017 Aug;29(4):414-421. 10.5021/ad.2017.29.4.414.
Influence of Repeated Senna Laxative Use on Skin Barrier Function in Mice
- Affiliations
-
- 1Laboratory of Community Pharmacy, Gifu Pharmaceutical University, Gifu, Japan.
- 2Department of Pharmaceutical Sciences, Faculty of Pharmaceutical Sciences, Suzuka University of Medical Science, Mie, Japan. hiramoto@suzuka-u.ac.jp
- KMID: 2388938
- DOI: http://doi.org/10.5021/ad.2017.29.4.414
Abstract
- BACKGROUND
Senna, one of the major stimulant laxatives, is widely used for treating constipation. Chronic senna use has been reported to be associated with colonic disorders such as melanosis coli and/or epithelial hyperplasia. However, there is no obvious information on the influence of chronic senna use on organs except for the intestine.
OBJECTIVE
To clarify the influence of senna laxative use on skin barrier function by repeated senna administration.
METHODS
Eight-week-old male hairless mice received senna (10 mg/kg/day) for 21 days. After administration, we evaluated transepidermal water loss (TEWL), and investigated the biomarkers in plasma and skin using protein analysis methods.
RESULTS
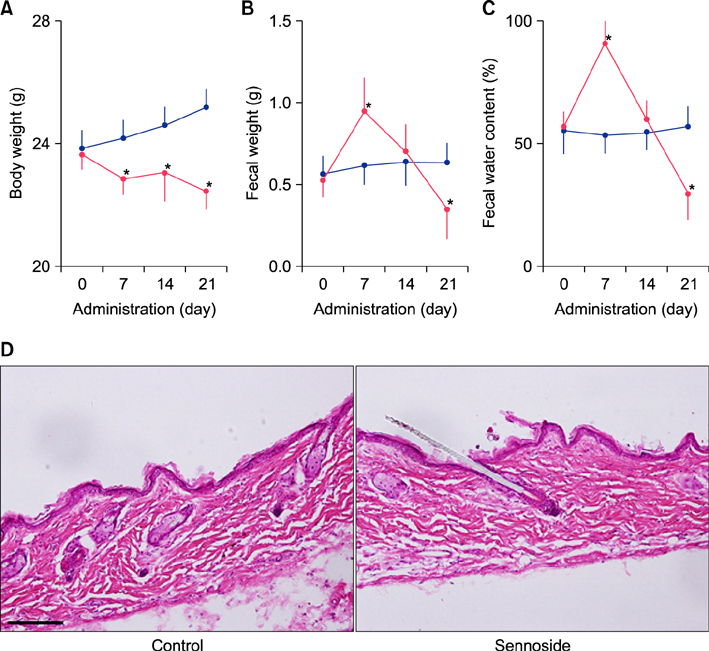
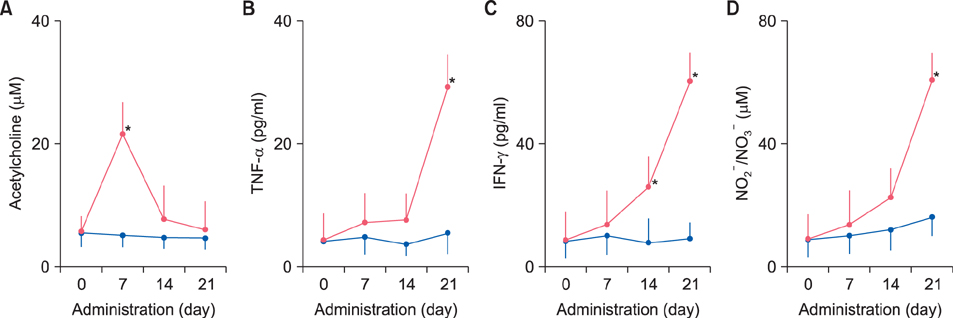
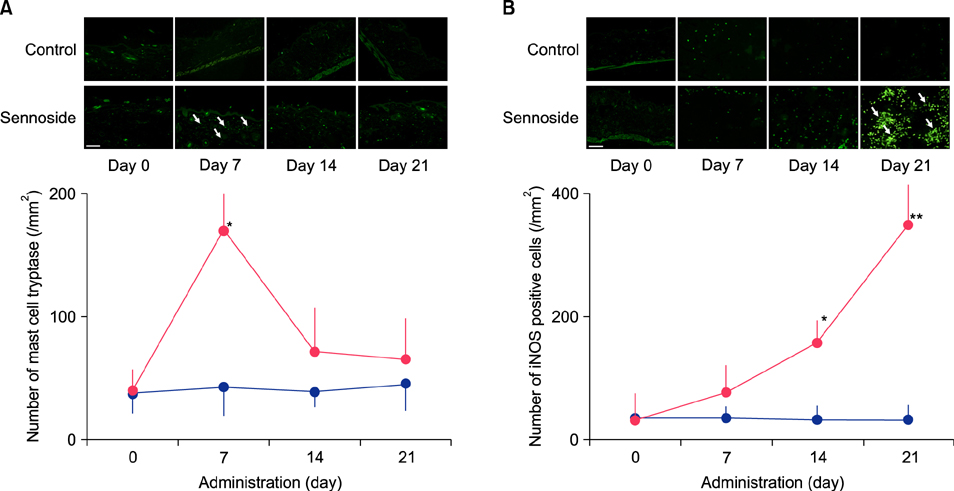
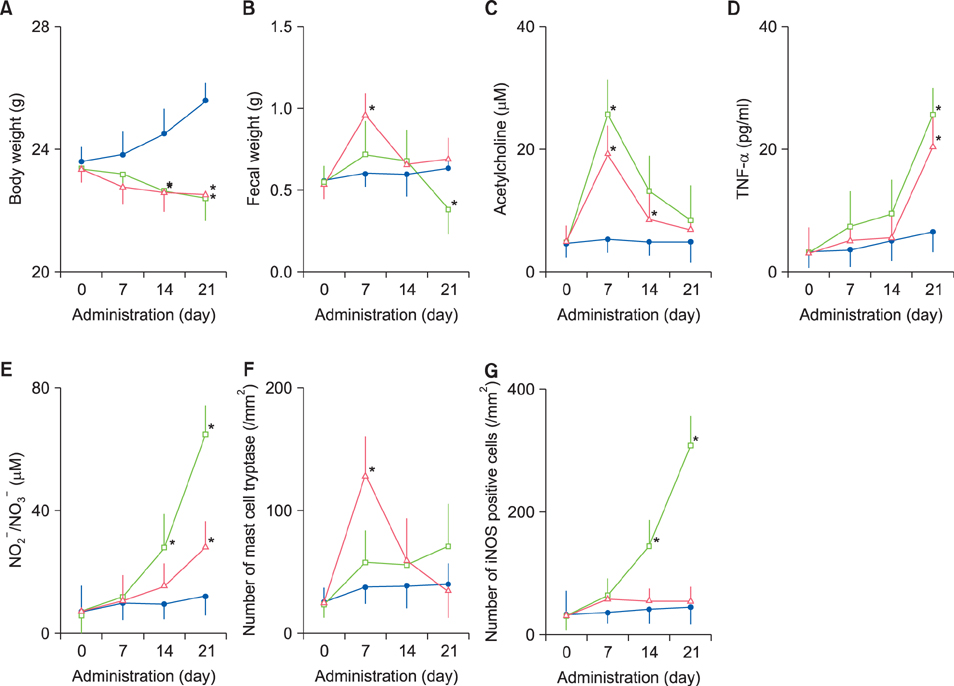
Fecal water content on day seven was significantly increased; however, on day 21, it was significantly decreased after repeated senna administration. In the senna-administered group, TEWL was significantly higher compared to the control on days seven and 21. Plasma acetylcholine concentration and NO2 −/NO3 − were increased on days seven and 21, respectively. In skin, tryptase-positive mast cells and inducible nitric oxide synthase (iNOS)-positive cells were increased on days seven and 21, respectively. The increase of TEWL on days seven and 21 was suppressed by the administration of atropine and N(G)-nitro-L-arginine methyl ester, respectively.
CONCLUSION
It was suggested that diarrhea or constipation induced by repeated senna administration caused the impairment of skin barrier function. There is a possibility that this impaired skin barrier function occurred due to degranulation of mast cells via cholinergic signals or oxidative stress derived from iNOS.
Keyword
MeSH Terms
-
Acetylcholine
Animals
Atropine
Biomarkers
Colon
Constipation
Diarrhea
Humans
Hyperplasia
Intestines
Laxatives
Male
Mast Cells
Melanosis
Mice*
Mice, Hairless
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase Type II
Oxidative Stress
Plasma
Senna Extract
Skin*
Water
Acetylcholine
Atropine
Biomarkers
Laxatives
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase Type II
Senna Extract
Water
Figure
Reference
-
1. Wald A, Scarpignato C, Mueller-Lissner S, Kamm MA, Hinkel U, Helfrich I, et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther. 2008; 28:917–930.
Article2. Morales MA, Hernandez D, Bustamante S, Bachiller I, Rojas A. Is senna laxative use associated to cathartic colon, genotoxicity, or carcinogenicity? J Toxicol. 2009; 2009:287247.
Article3. Yamate Y, Hiramoto K, Yokoyama S, Ooi K. Immunological changes in the intestines and skin after senna administration. Pharm Biol. 2015; 53:913–920.
Article4. Matsui T, Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol. 2015; 27:269–280.
Article5. Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009; 206:2937–2946.
Article6. Huang BL, Chandra S, Shih DQ. Skin manifestations of inflammatory bowel disease. Front Physiol. 2012; 3:13.
Article7. Yokoyama S, Hiramoto K, Koyama M, Ooi K. Skin disruption is associated with indomethacin-induced small intestinal injury in mice. Exp Dermatol. 2014; 23:659–663.
Article8. Yokoyama S, Hiramoto K, Koyama M, Ooi K. Impaired skin barrier function in mice with colon carcinoma induced by azoxymethane and dextran sodium sulfate. Biol Pharm Bull. 2015; 38:947–950.
Article9. Yokoyama S, Hiramoto K, Koyama M, Ooi K. Impairment of skin barrier function via cholinergic signal transduction in a DSS-induced colitis mouse model. Exp Dermatol. 2015; 24:779–784.
Article10. Frieling T, Rupprecht C, Schemann M. Rhein stimulates electrogenic chloride secretion by activation of submucosal neurons in guinea pig colon. Pharmacology. 1993; 47:Suppl 1. 70–76.
Article11. Blandina P, Fantozzi R, Mannaioni PF, Masini E. Characteristics of histamine release evoked by acetylcholine in isolated rat mast cells. J Physiol. 1980; 301:281–293.
Article12. Radosa J, Dyck W, Goerdt S, Kurzen H. The cholinergic system in guttate psoriasis with special reference to mast cells. Exp Dermatol. 2011; 20:677–679.
Article13. Masini E, Fantozzi R, Conti A, Blandina P, Brunelleschi S, Mannaioni PF. Mast cell heterogeneity in response to cholinergic stimulation. Int Arch Allergy Appl Immunol. 1985; 77:184–185.
Article14. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006; 86:1309–1379.
Article15. Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007; 39:125–135.
Article16. Kageyama-Yahara N, Suehiro Y, Yamamoto T, Kadowaki M. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem Biophys Res Commun. 2008; 377:321–325.
Article17. Sudheer PS, Hall JE, Donev R, Read G, Rowbottom A, Williams PE. Nicotinic acetylcholine receptors on basophils and mast cells. Anaesthesia. 2006; 61:1170–1174.
Article18. Buchli R, Ndoye A, Rodriguez JG, Zia S, Webber RJ, Grando SA. Human skin fibroblasts express m2, m4, and m5 subtypes of muscarinic acetylcholine receptors. J Cell Biochem. 1999; 74:264–277.
Article19. Kurzen H, Henrich C, Booken D, Poenitz N, Gratchev A, Klemke CD, et al. Functional characterization of the epidermal cholinergic system in vitro. J Invest Dermatol. 2006; 126:2458–2472.
Article20. Nadal SR, Calore EE, Manzione CR, Puga FR, Perez NM. Effects of long-term administration of Senna occidentalis seeds in the large bowel of rats. Pathol Res Pract. 2003; 199:733–737.
Article21. Mengs U, Mitchell J, McPherson S, Gregson R, Tigner J. A 13-week oral toxicity study of senna in the rat with an 8-week recovery period. Arch Toxicol. 2004; 78:269–275.
Article22. Schreiber O, Petersson J, Walden T, Ahl D, Sandler S, Phillipson M, et al. iNOS-dependent increase in colonic mucus thickness in DSS-colitic rats. PLoS One. 2013; 8:e71843.
Article23. Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014; 7:113–120.
Article24. Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993; 177:1779–1784.
Article25. Colon AL, Menchen LA, Hurtado O, De Cristobal J, Lizasoain I, Leza JC, et al. Implication of TNF-alpha converttase (TACE/ADAM17) in inducible nitric oxide synthase expression and inflammation in an experimental model of colitis. Cytokine. 2001; 16:220–226.
Article26. Mitchell JM, Mengs U, McPherson S, Zijlstra J, Dettmar P, Gregson R, et al. An oral carcinogenicity and toxicity study of senna (Tinnevelly senna fruits) in the rat. Arch Toxicol. 2006; 80:34–44.
Article27. Surh I, Brix A, French JE, Collins BJ, Sanders JM, Vallant M, et al. Toxicology and carcinogenesis study of senna in C3B6.129F1-Trp53 tm1Brd N12 haploinsufficient mice. Toxicol Pathol. 2013; 41:770–778.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differences in the Recovery Rate after Perturbation of Epidermal Barrier by Means of Acetone Treatment and Tape-Stripping Technique
- The Influence of Physiologic Lipid Containing Moisturizer on the Normal Skin Barrier
- The Ultrastructural Changes of Stratum Corneum Lipids after Application of Oleic Acid in Propylene Glycol
- Atopic Dermatitis and Epidermal Barrier
- Acute Modulations in Stratum Corneum Permeability Barrier Function Affect Claudin Expression and Epidermal Tight Junction Function via Changes of Epidermal Calcium Gradient