Clin Endosc.
2017 May;50(3):279-286. 10.5946/ce.2016.107.
External Validation of the Endoscopic Features of Sessile Serrated Adenomas in Expert and Trainee Colonoscopists
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Gastrointestinal Cancer Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. diksmc.park@samsung.com
- 2Department of Pathology, Gastrointestinal Cancer Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2388825
- DOI: http://doi.org/10.5946/ce.2016.107
Abstract
- BACKGROUND/AIMS
It is unclear whether the endoscopic features of sessile serrated adenomas (SSAs) would be useful to trainee colonoscopists to predict SSA. Therefore, the present study aimed to identify features that expert and trainee colonoscopists can use to independently and reliably predict SSA by using high-resolution white-light endoscopy.
METHODS
Endoscopic images of 81 polyps (39 SSAs, 22 hyperplastic polyps, and 20 tubular adenomas) from 43 patients were retrospectively evaluated by 10 colonoscopists (four experts and six trainees). Eight endoscopic features of SSAs were assessed for each polyp.
RESULTS
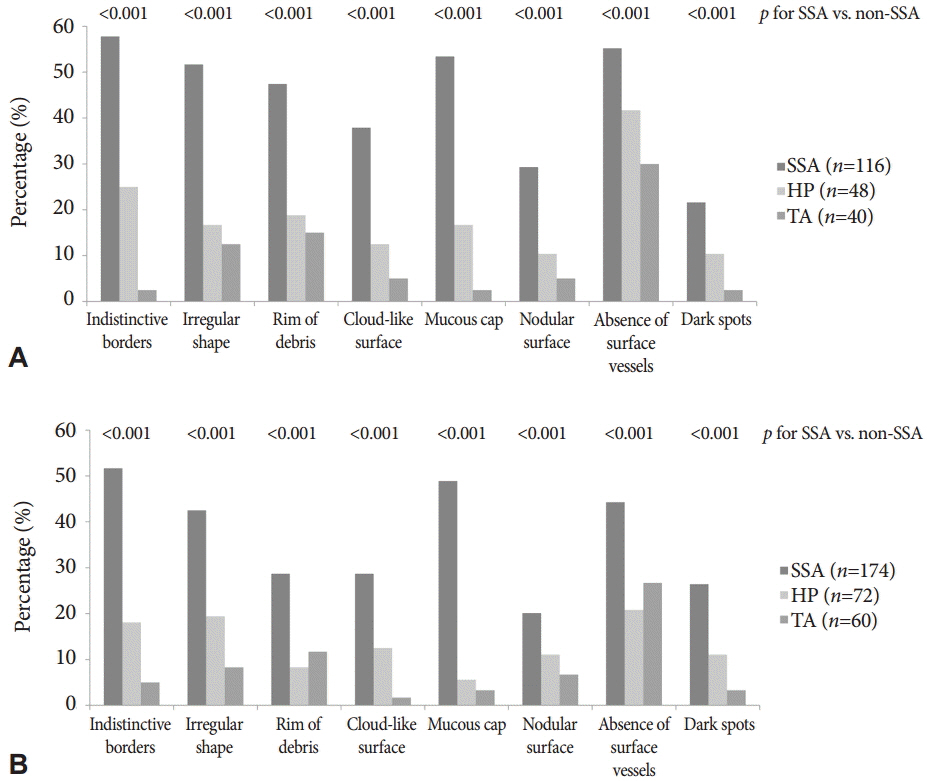
According to multivariable analysis, a mucous cap (odds ratio [OR], 10.44; 95% confidence interval [CI], 5.72 to 19.07), indistinctive borders (OR, 4.21; 95% CI, 2.74 to 7.16), dark spots (OR, 3.64; 95% CI, 1.89 to 7.00), and cloud-like surface (OR, 2.43; 95% CI, 1.27 to 4.668) were independent predictors of SSAs. Among these, a mucous cap, indistinctive borders, and cloud-like surface showed moderate interobserver agreement (mean κ>0.40) among experts and trainees. When ≥1 of the three predictors was observed, the sensitivity and specificity for diagnosing SSAs were 79.0% and 81.4%, respectively.
CONCLUSIONS
Colonoscopy trainees and experts can use several specific endoscopic features to independently and reliably predict SSAs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Sessile Serrated Adenoma; the Hard-to-Catch Culprit of Interval Cancer
Suk Pyo Shin
Clin Endosc. 2017;50(3):215-216. doi: 10.5946/ce.2017.052.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Vleugels JL, van Lanschot MC, Dekker E. Colorectal cancer screening by colonoscopy: putting it into perspective. Dig Endosc. 2016; 28:250–259.
Article3. Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014; 348:g2467.
Article4. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013; 369:1095–1105.
Article5. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012; 366:687–696.
Article6. Lin OS, Kozarek RA, Cha JM. Impact of sigmoidoscopy and colonoscopy on colorectal cancer incidence and mortality: an evidence-based review of published prospective and retrospective studies. Intest Res. 2014; 12:268–274.
Article7. Brenner H, Chang-Claude J, Rickert A, Seiler CM, Hoffmeister M. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case-control study. J Clin Oncol. 2012; 30:2969–2976.
Article8. Kim CJ, Jung YS, Park JH, et al. Prevalence, clinicopathologic characteristics, and predictors of interval colorectal cancers in Korean population. Intest Res. 2013; 11:178–183.
Article9. Cha JM. Colonoscopy quality is the answer for the emerging issue of interval cancer. Intest Res. 2014; 12:110–116.
Article10. le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014; 63:957–963.
Article11. Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011; 42:1–10.
Article12. Holme O, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut. 2015; 64:929–936.
Article13. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010; 138:2088–2100.
Article14. Tadepalli US, Feihel D, Miller KM, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video). Gastrointest Endosc. 2011; 74:1360–1368.
Article15. Hazewinkel Y, López-Cerón M, East JE, et al. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013; 77:916–924.
Article16. JE IJ, Bastiaansen BA, van Leerdam ME, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2016; 65:963–970.
Article17. Yamashina T, Takeuchi Y, Uedo N, et al. Diagnostic features of sessile serrated adenoma/polyps on magnifying narrow band imaging: a prospective study of diagnostic accuracy. J Gastroenterol Hepatol. 2015; 30:117–123.
Article18. Yamada M, Sakamoto T, Otake Y, et al. Investigating endoscopic features of sessile serrated adenomas/polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2015; 82:108–117.
Article19. Ignjatovic A, Thomas-Gibson S, East JE, et al. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc. 2011; 73:128–133.
Article20. Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010; 72:572–576.
Article21. Ng SC, Lau JY. Narrow-band imaging in the colon: limitations and potentials. J Gastroenterol Hepatol. 2011; 26:1589–1596.
Article22. Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis. In : Bosman FT, editor. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer;2010. p. 160–165.23. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174.
Article24. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley;2000. p. 392.25. Yamada A, Notohara K, Aoyama I, et al. Endoscopic features of sessile serrated adenoma and other serrated colorectal polyps. Hepatogastroenterology. 2011; 58:45–51.26. Jaramillo E, Tamura S, Mitomi H. Endoscopic appearance of serrated adenomas in the colon. Endoscopy. 2005; 37:254–260.
Article27. Bordacahar B, Barret M, Terris B, et al. Sessile serrated adenoma: from identification to resection. Dig Liver Dis. 2015; 47:95–102.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sessile Serrated Adenomas: How to Detect, Characterize and Resect
- Sessile Serrated Adenoma with High-grade Dysplasia
- Characteristics and outcomes of endoscopically resected colorectal cancers that arose from sessile serrated adenomas and traditional serrated adenomas
- Clinical Features of Colorectal Serrated Adenomas
- Serrated Adenoma with Adenocarcinoma of Stomach Treated by Endoscopic Submucosal Dissection