Korean J Physiol Pharmacol.
2017 Sep;21(5):475-485. 10.4196/kjpp.2017.21.5.475.
RhGLP-1 (7–36) protects diabetic rats against cerebral ischemia-reperfusion injury via up-regulating expression of Nrf2/HO-1 and increasing the activities of SOD
- Affiliations
-
- 1Department of Pharmacy, Peking University People's Hospital, Beijing 100044, China.
- 2Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, China.
- 3Department of Pharmacy, Guizhou Orthopedics Hospital, Guizhou 550002, China.
- 4Department of Pharmacy, Xuzhou Medical University, Xuzhou 221004, China. yinxx@xzhmu.edu.cn
- 5Department of Pharmacy, Mawangdui Hospital, Changsha 410016, China.
- 6Department of oncology, Harrison International Peace Hospital, Hengshui 053000, China.
- KMID: 2388693
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.475
Abstract
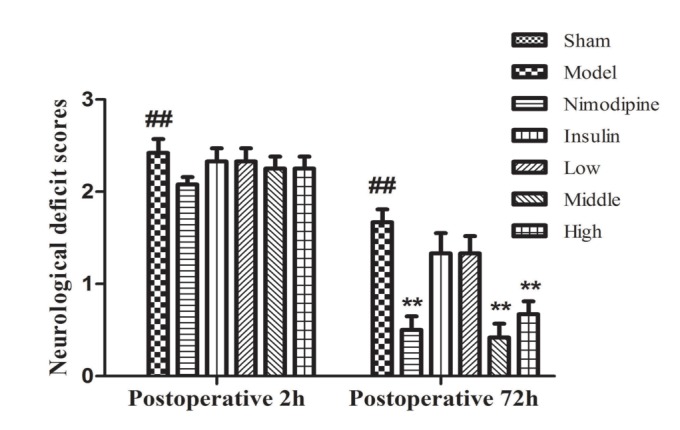
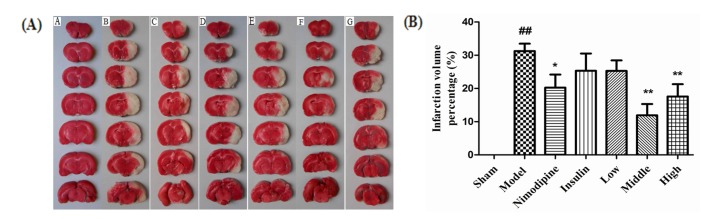
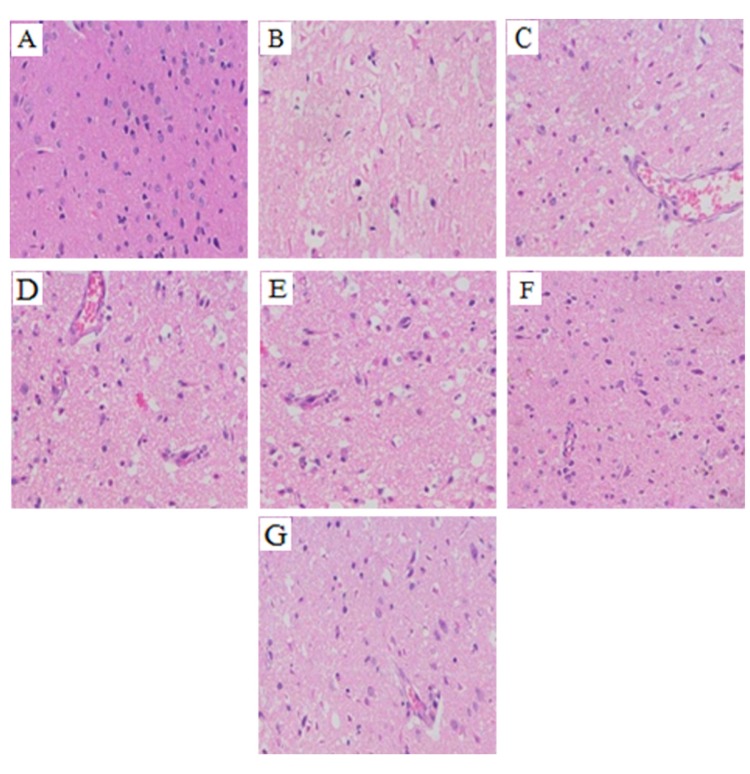
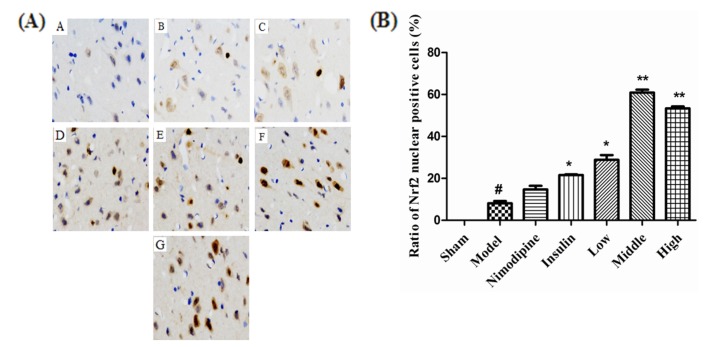
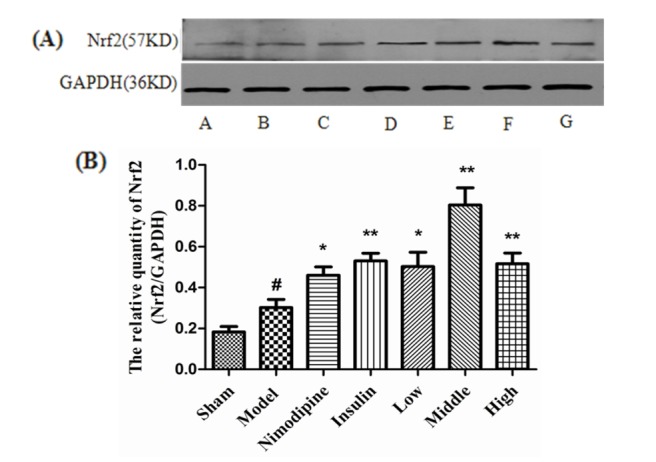
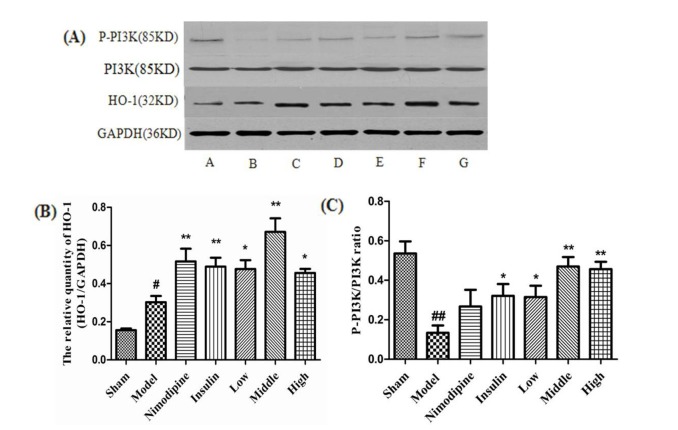
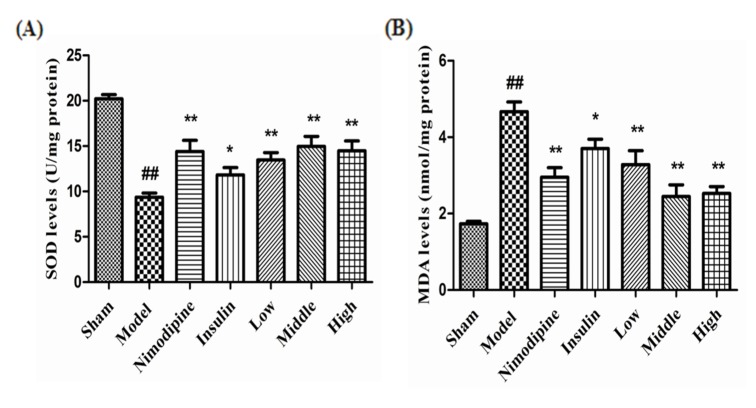
- The present study aimed to explore the neuroprotective effect and possible mechanisms of rhGLP-1 (7-36) against transient ischemia/reperfusion injuries induced by middle cerebral artery occlusion (MCAO) in type 2 diabetic rats. First, diabetic rats were established by a combination of a high-fat diet and low-dose streptozotocin (STZ) (30 mg/kg, intraperitoneally). Second, they were subjected to MCAO for 2 h, then treated with rhGLP-1 (7-36) (10, 20, 40 µg/kg i.p.) at the same time of reperfusion. In the following 3 days, they were injected with rhGLP-1 (7-36) at the same dose and route for three times each day. After 72 h, hypoglycemic effects were assessed by blood glucose changes, and neuroprotective effects were evaluated by neurological deficits, infarct volume and histomorphology. Mechanisms were investigated by detecting the distribution and expression of the nuclear factor erythroid-derived factor 2 related factor 2 (Nrf2) in ischemic brain tissue, the levels of phospho-PI3 kinase (PI3K)/PI3K ratio and heme-oxygenase-1 (HO-l), as well as the activities of superoxide dismutase (SOD) and the contents of malondialdehyde (MDA). Our results showed that rhGLP-1 (7-36) significantly reduced blood glucose and infarction volume, alleviated neurological deficits, enhanced the density of surviving neurons and vascular proliferation. The nuclear positive cells ratio and expression of Nrf2, the levels of P-PI3K/PI3K ratio and HO-l increased, the activities of SOD increased and the contents of MDA decreased. The current results indicated the protective effect of rhGLP-1 (7-36) in diabetic rats following MCAO/R that may be concerned with reducing blood glucose, up-regulating expression of Nrf2/HO-1 and increasing the activities of SOD.
MeSH Terms
-
Animals
Blood Glucose
Brain
Diet, High-Fat
Hypoglycemic Agents
Infarction
Infarction, Middle Cerebral Artery
Malondialdehyde
Neurons
Neuroprotective Agents
Phosphotransferases
Rats*
Reperfusion
Reperfusion Injury*
Streptozocin
Superoxide Dismutase
Blood Glucose
Hypoglycemic Agents
Malondialdehyde
Neuroprotective Agents
Phosphotransferases
Streptozocin
Superoxide Dismutase
Figure
Reference
-
1. Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP, Gebel J, Shukla R, Broderick JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005; 28:355–359. PMID: 15677792.2. Zhao D, Liu J, Wang W, Zeng Z, Cheng J, Liu J, Sun J, Wu Z. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008; 39:1668–1674. PMID: 18309149.3. Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007; 87:179–197. PMID: 17521716.
Article4. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010; 67:181–198. PMID: 20670828.
Article5. Briyal S, Gulati K, Gulati A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res. 2012; 1427:23–34. PMID: 22055454.
Article6. Watanabe Y, Kawai K, Ohashi S, Yokota C, Suzuki S, Yamashita K. Structure-activity relationships of glucagon-like peptide-1(7-36) amide: insulinotropic activities in perfused rat pancreases, and receptor binding and cyclic AMP production in RINm5F cells. J Endocrinol. 1994; 140:45–52. PMID: 8138751.7. Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983; 302:716–718. PMID: 6835407.
Article8. Dunphy JL, Taylor RG, Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol. 1998; 141:179–186. PMID: 9723898.9. Parsons GB, Souza DW, Wu H, Yu D, Wadsworth SG, Gregory RJ, Armentano D. Ectopic expression of glucagon-like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007; 14:38–48. PMID: 16929351.
Article10. Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004; 145:2653–2659. PMID: 15044356.
Article11. Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000; 49:741–748. PMID: 10905482.
Article12. Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5'-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003; 144:1444–1455. PMID: 12639928.
Article13. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007; 132:2131–2157. PMID: 17498508.
Article14. Puddu A, Sanguineti R, Durante A, Nencioni A, Mach F, Montecucco F, Viviani GL. Glucagon-like peptide-1 triggers protective pathways in pancreatic beta-cells exposed to glycated serum. Mediators Inflamm. 2013; 2013:317120. PMID: 23737644.
Article15. Fernández-Millán E, Martín MA, Goya L, Lizárraga-Mollinedo E, Escrivá F, Ramos S, Álvarez C. Glucagon-like peptide-1 improves beta-cell antioxidant capacity via extracellular regulated kinases pathway and Nrf2 translocation. Free Radic Biol Med. 2016; 95:16–26. PMID: 26968794.
Article16. Zhang H, Xiong Z, Wang J, Zhang S, Lei L, Yang L, Zhang Z. Glucagon-like peptide-1 protects cardiomyocytes from advanced oxidation protein product-induced apoptosis via the PI3K/Akt/Bad signaling pathway. Mol Med Rep. 2016; 13:1593–1601. PMID: 26717963.
Article17. Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009; 106:1285–1290. PMID: 19164583.
Article18. Teramoto S, Miyamoto N, Yatomi K, Tanaka Y, Oishi H, Arai H, Hattori N, Urabe T. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2011; 31:1696–1705. PMID: 21487412.
Article19. Sato K, Kameda M, Yasuhara T, Agari T, Baba T, Wang F, Shinko A, Wakamori T, Toyoshima A, Takeuchi H, Sasaki T, Sasada S, Kondo A, Borlongan CV, Matsumae M, Date I. Neuroprotective effects of liraglutide for stroke model of rats. Int J Mol Sci. 2013; 14:21513–21524. PMID: 24177570.
Article20. Briyal S, Shah S, Gulati A. Neuroprotective and anti-apoptotic effects of liraglutide in the rat brain following focal cerebral ischemia. Neuroscience. 2014; 281:269–281. PMID: 25301749.
Article21. Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005; 28:1083–1091. PMID: 15855571.
Article22. Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004; 27:1915–1921. PMID: 15277417.
Article23. Butler PC, Dry S, Elashoff R. GLP-1-based therapy for diabetes: what you do not know can hurt you. Diabetes Care. 2010; 33:453–455. PMID: 20103562.
Article24. Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010; 33:428–433. PMID: 20103558.
Article25. Zhao L, Xu J, Wang Q, Qian Z, Feng W, Yin X, Fang Y. Protective effect of rhGLP-1 (7-36) on brain ischemia/reperfusion damage in diabetic rats. Brain Res. 2015; 1602:153–159. PMID: 25601009.
Article26. Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005; 52:313–320. PMID: 15979893.
Article27. Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989; 20:1037–1043. PMID: 2756535.
Article28. Li F, Irie K, Anwer MS, Fisher M. Delayed triphenyltetrazolium chloride staining remains useful for evaluating cerebral infarct volume in a rat stroke model. J Cereb Blood Flow Metab. 1997; 17:1132–1135. PMID: 9346439.
Article29. Hutchinson DS, Summers RJ, Bengtsson T. Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol Ther. 2008; 119:291–310. PMID: 18606183.
Article30. Rossi MC, Nicolucci A. Liraglutide in type 2 diabetes: from pharmacological development to clinical practice. Acta Biomed. 2009; 80:93–101. PMID: 19848045.31. Kimura R, Okouchi M, Fujioka H, Ichiyanagi A, Ryuge F, Mizuno T, Imaeda K, Okayama N, Kamiya Y, Asai K, Joh T. Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience. 2009; 162:1212–1219. PMID: 19463904.
Article32. Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer's disease. Behav Brain Res. 2009; 205:265–271. PMID: 19573562.
Article33. Lu ZQ, Deng YJ, Lu JX. Effect of aloe polysaccharide on caspase-3 expression following cerebral ischemia and reperfusion injury in rats. Mol Med Rep. 2012; 6:371–374. PMID: 22641427.
Article34. Chang XB, Fan XN, Wang S, Yang S, Yang X, Zhang YN, Shi XM. Influence of acupuncture on neural movement function in rats with middle cerebral artery occlusion-a randomized controlled trial. J Tradit Chin Med. 2012; 32:105–109. PMID: 22594112.
Article35. Pham V, Albiston AL, Downes CE, Wong CH, Diwakarla S, Ng L, Lee S, Crack PJ, Chai SY. Insulin-regulated aminopeptidase deficiency provides protection against ischemic stroke in mice. J Neurotrauma. 2012; 29:1243–1248. PMID: 21895534.
Article36. Fang Y, Chai D, Zheng Z, Wang X. Pharmacokinetics of recombined human glucagonlike peptide-1 (7-36) in Chinese healthy volunteers. Chin J Clin Pharmacol. 2006; 22:250–253.37. Smolen HJ, Murphy DR, Gahn JC, Yu X, Curtis BH. The evaluation of clinical and cost outcomes associated with earlier initiation of insulin in patients with type 2 diabetes mellitus. J Manag Care Spec Pharm. 2014; 20:968–984. PMID: 25166296.
Article38. Taya K, Watanabe Y, Kobayashi H, Fujiwara M. Nimodipine improves the disruption of spatial cognition induced by cerebral ischemia. Physiol Behav. 2000; 70:19–25. PMID: 10978473.
Article39. Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994; 91:9926–9930. PMID: 7937919.
Article40. Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011; 589:4125–4136. PMID: 21646410.
Article41. Tanaka N, Ikeda Y, Ohta Y, Deguchi K, Tian F, Shang J, Matsuura T, Abe K. Expression of Keap1-Nrf2 system and antioxidative proteins in mouse brain after transient middle cerebral artery occlusion. Brain Res. 2011; 1370:246–253. PMID: 21075092.
Article42. Rojo AI, Innamorato NG, Martín-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010; 58:588–598. PMID: 19908287.
Article43. Innamorato NG, Rojo AI, García-Yagüe AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008; 181:680–689. PMID: 18566435.
Article44. Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007; 147:53–59. PMID: 17507167.
Article45. Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009; 11:e17. PMID: 19490732.
Article46. Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003; 278:37948–37956. PMID: 12842875.
Article47. Okouchi M, Okayama N, Alexander JS, Aw TY. NRF2-dependent glutamate-L-cysteine ligase catalytic subunit expression mediates insulin protection against hyperglycemia-induced brain endothelial cell apoptosis. Curr Neurovasc Res. 2006; 3:249–261. PMID: 17109620.
Article48. Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci U S A. 2006; 103:768–773. PMID: 16407140.49. Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005; 280:29158–29168. PMID: 15901726.
Article50. Ku BM, Joo Y, Mun J, Roh GS, Kang SS, Cho GJ, Choi WS, Kim HJ. Heme oxygenase protects hippocampal neurons from ethanol-induced neurotoxicity. Neurosci Lett. 2006; 405:168–171. PMID: 16857315.
Article51. Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007; 42:1838–1850. PMID: 17512463.
Article52. Cao Z, Zhu H, Zhang L, Zhao X, Zweier JL, Li Y. Antioxidants and phase 2 enzymes in cardiomyocytes: Chemical inducibility and chemoprotection against oxidant and simulated ischemia-reperfusion injury. Exp Biol Med (Maywood). 2006; 231:1353–1364. PMID: 16946404.
Article53. Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, Xiao X. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain Res. 2013; 1491:188–196. PMID: 23123706.
Article54. Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009; 1282:133–141. PMID: 19445907.
Article55. Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002; 22:2883–2892. PMID: 11940647.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Ischemic Preconditioning on the Phosphorylation of Akt and the Expression of SOD-1 in the Ischemic-reperfused Rat Skeletal Muscles
- The Changes of SOD, Catalase and GPX in Alloxan Induced Diabetic Pregnant Rats, Their Feurses and Neonates
- Effect of Allopurinol Pretreatment on Superoxide Dismutase Activity in Ischemia Reperfusion Injury to Skeletal Muscles of the Hindlimbs of the Rats
- Effects of Remote Ischemic Preconditioning on the Expressions of NOS and Akt in the Rat Myocardium
- Effect of Superoxide Dismutase and Dimethylthiourea on the Ultrastructure of Hepatocytes in Normothermic Hepatic Ischemia-Reperfusion Injury of Rats