Korean J Physiol Pharmacol.
2016 Mar;20(2):169-175. 10.4196/kjpp.2016.20.2.169.
High glucose and palmitate increases bone morphogenic protein 4 expression in human endothelial cells
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. drkwon@catholic.ac.kr
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Kyunggi-do 14647, Korea.
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 07345, Korea.
- 5Coulter Department of Biomedical Engineering at Georgia Tech and Emory University, Atlanta, GA 30322, USA.
- KMID: 2388673
- DOI: http://doi.org/10.4196/kjpp.2016.20.2.169
Abstract
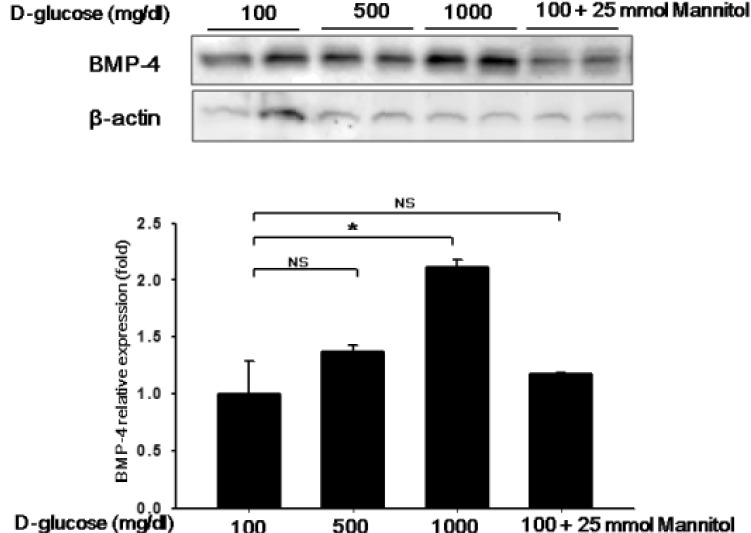
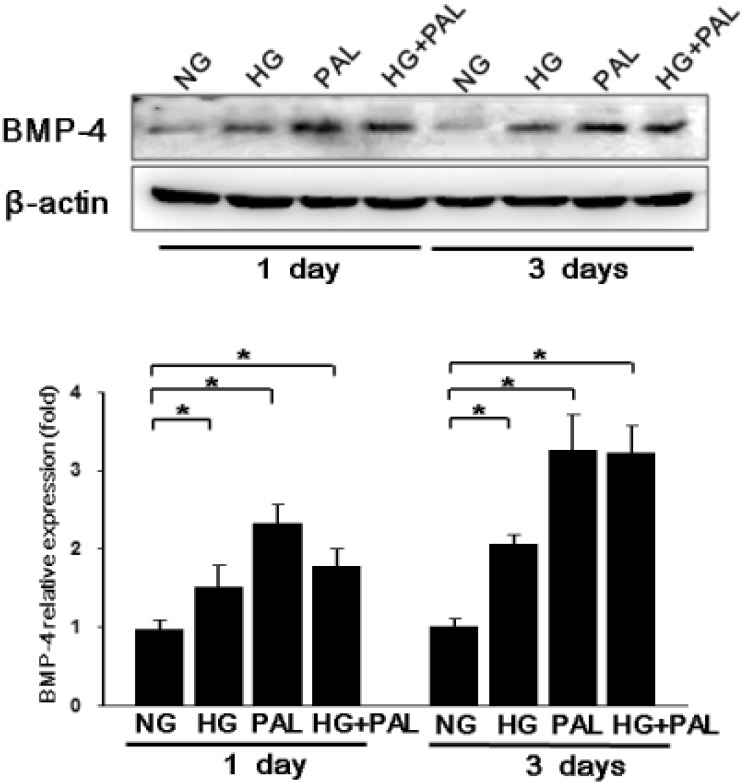
- Here, we investigated whether hyperglycemia and/or free fatty acids (palmitate, PAL) aff ect the expression level of bone morphogenic protein 4 (BMP4), a proatherogenic marker, in endothelial cells and the potential role of BMP4 in diabetic vascular complications. To measure BMP4 expression, human umbilical vein endothelial cells (HUVECs) were exposed to high glucose concentrations and/or PAL for 24 or 72 h, and the effects of these treatments on the expression levels of adhesion molecules and reactive oxygen species (ROS) were examined. BMP4 loss-of-function status was achieved via transfection of a BMP4-specific siRNA. High glucose levels increased BMP4 expression in HUVECs in a dose-dependent manner. PAL potentiated such expression. The levels of adhesion molecules and ROS production increased upon treatment with high glucose and/or PAL, but this eff ect was negated when BMP4 was knocked down via siRNA. Signaling of BMP4, a proinflammatory and pro-atherogenic cytokine marker, was increased by hyperglycemia and PAL. BMP4 induced the expression of infl ammatory adhesion molecules and ROS production. Our work suggests that BMP4 plays a role in atherogenesis induced by high glucose levels and/or PAL.
MeSH Terms
Figure
Reference
-
1. Wong WT, Tian XY, Chen Y, Leung FP, Liu L, Lee HK, Ng CF, Xu A, Yao X, Vanhoutte PM, Tipoe GL, Huang Y. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res. 2010; 107:984–991. PMID: 20724703.2. Cai J, Pardali E, Sánchez-Duffhues G, ten Dijke P. BMP signaling in vascular diseases. FEBS Lett. 2012; 586:1993–2002. PMID: 22710160.
Article3. Maciel TT, Kempf H, Campos AH. Targeting bone morphogenetic protein signaling on renal and vascular diseases. Curr Opin Nephrol Hypertens. 2010; 19:26–31. PMID: 19823085.
Article4. Boström KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011; 108:446–457. PMID: 21193740.
Article5. Csiszar A, Labinskyy N, Smith KE, Rivera A, Bakker EN, Jo H, Gardner J, Orosz Z, Ungvari Z. Downregulation of bone morphogenetic protein 4 expression in coronary arterial endothelial cells: role of shear stress and the cAMP/protein kinase A pathway. Arterioscler Thromb Vasc Biol. 2007; 27:776–782. PMID: 17272757.6. Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassègue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004; 95:773–779. PMID: 15388638.
Article7. Koga M, Engberding N, Dikalova AE, Chang KH, Seidel-Rogol B, Long JS, Lassègue B, Jo H, Griendling KK. The bone morphogenic protein inhibitor, noggin, reduces glycemia and vascular inflammation in db/db mice. Am J Physiol Heart Circ Physiol. 2013; 305:H747–H755. PMID: 23812391.
Article8. Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4-induced inflammation and atherosclerosis. Antioxid Redox Signal. 2006; 8:1609–1619. PMID: 16987015.9. Csiszar A, Lehoux S, Ungvari Z. Hemodynamic forces, vascular oxidative stress, and regulation of BMP-2/4 expression. Antioxid Redox Signal. 2009; 11:1683–1697. PMID: 19320562.
Article10. Csiszar A, Labinskyy N, Jo H, Ballabh P, Ungvari Z. Differential proinflammatory and prooxidant effects of bone morphogenetic protein-4 in coronary and pulmonary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2008; 295:H569–H577. PMID: 18539760.
Article11. Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010; 298:F125–F132. PMID: 19864304.
Article12. Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007; 148:160–165. PMID: 17023526.
Article13. Hou Q, Lei M, Hu K, Wang M. The effects of high glucose levels on reactive oxygen species-induced apoptosis and involved signaling in human vascular endothelial cells. Cardiovasc Toxicol. 2015; 15:140–146. PMID: 25158671.
Article14. Hu J, Liu J, Kwok MW, Wong RH, Huang Y, Wan S. Bone morphogenic protein-4 contributes to venous endothelial dysfunction in patients with diabetes undergoing coronary revascularization. Ann Thorac Surg. 2013; 95:1331–1339. PMID: 23522199.
Article15. Zhang Y, Liu J, Tian XY, Wong WT, Chen Y, Wang L, Luo J, Cheang WS, Lau CW, Kwan KM, Wang N, Yao X, Huang Y. Inhibition of bone morphogenic protein 4 restores endothelial function in db/db diabetic mice. Arterioscler Thromb Vasc Biol. 2014; 34:152–159. PMID: 24202302.16. Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006; 116:1071–1080. PMID: 16528409.
Article17. Son JW, Jang EH, Kim MK, Baek KH, Song KH, Yoon KH, Cha BY, Son HY, Lee KW, Jo H, Kwon HS. Serum BMP-4 levels in relation to arterial stiffness and carotid atherosclerosis in patients with Type 2 diabetes. Biomark Med. 2011; 5:827–835. PMID: 22103619.
Article18. Zhu P, Chen G, You T, Yao J, Jiang Q, Lin X, Shen X, Qiao Y, Lin L. High FFA-induced proliferation and apoptosis in human umbilical vein endothelial cell partly through Wnt/beta-catenin signal pathway. Mol Cell Biochem. 2010; 338:123–131. PMID: 19967550.19. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000; 49:1939–1945. PMID: 11078463.
Article20. Upton PD, Long L, Trembath RC, Morrell NW. Functional characterization of bone morphogenetic protein binding sites and Smad1/5 activation in human vascular cells. Mol Pharmacol. 2008; 73:539–552. PMID: 17989347.
Article21. Wu J, Yu Z, Su D. BMP4 protects rat pulmonary arterial smooth muscle cells from apoptosis by PI3K/AKT/Smad1/5/8 signaling. Int J Mol Sci. 2014; 15:13738–13754. PMID: 25110865.
Article22. Zhang M, Zhou SH, Zhao S, Li XP, Liu LP, Shen XQ. Pioglitazone can downregulate bone morphogenetic protein-2 expression induced by high glucose in human umbilical vein endothelial cells. Pharmacology. 2008; 81:312–316. PMID: 18311072.
Article23. Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006; 168:629–638. PMID: 16436676.
Article24. Kim CW, Song H, Kumar S, Nam D, Kwon HS, Chang KH, Son DJ, Kang DW, Brodie SA, Weiss D, Vega JD, Alberts-Grill N, Griendling K, Taylor WR, Jo H. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013; 33:1350–1359. PMID: 23559633.
Article25. Chung JH, Jeon HJ, Hong SY, Lee da L, Lee KH, Kim SH, Han YS, Manabe I, Miller YI, Lee SH. Palmitate promotes the paracrine effects of macrophages on vascular smooth muscle cells: the role of bone morphogenetic proteins. PLoS One. 2012; 7:e29100. PMID: 22363399.
Article26. Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009; 29:1370–1375. PMID: 19542021.27. Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001; 107:1255–1262. PMID: 11375415.
Article28. Xu S, He Y, Vokurkova M, Touyz RM. Endothelial cells negatively modulate reactive oxygen species generation in vascular smooth muscle cells: role of thioredoxin. Hypertension. 2009; 54:427–433. PMID: 19564543.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on Role of Neutrophil in Endothelial Cell Injury under High Glucose Condition
- Effect and Mechanism of High Glucose Level on the Expression of an Adhesion Protein, beta ig-h3, and Cellular Function in Endothelial Cells
- AM1638, a GPR40-Full Agonist, Inhibited Palmitate- Induced ROS Production and Endoplasmic Reticulum Stress, Enhancing HUVEC Viability in an NRF2-Dependent Manner
- Decreased Expression and Induced Nucleocytoplasmic Translocation of Pancreatic and Duodenal Homeobox 1 in INS-1 Cells Exposed to High Glucose and Palmitate
- Expression of cartilage derived morphogenic protein in pleomorphic adenoma