Cancer Res Treat.
2017 Jul;49(3):759-765. 10.4143/crt.2016.371.
Efficacy of Chemotherapy in Patients with Unresectable or Metastatic Pancreatic Acinar Cell Carcinoma: Potentially Improved Efficacy with Oxaliplatin-Containing Regimen
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. changhm@amc.seoul.kr
- 2Department of Internal Medicine, Hallym University Medical Center, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2388321
- DOI: http://doi.org/10.4143/crt.2016.371
Abstract
- PURPOSE
Pancreatic acinar cell carcinoma (ACC) is a rare cancer of the exocrine pancreas. Because of its rare incidence, the efficacy of chemotherapy in this patient population has been largely unknown. Therefore, we retrospectively analyzed the outcomes of patients with advanced pancreatic ACC who received chemotherapy.
MATERIALS AND METHODS
Between January 1997 and March 2015, 15 patients with unresectable or metastatic pancreatic ACC who received systemic chemotherapy were identified in Asan Medical Center, Korea.
RESULTS
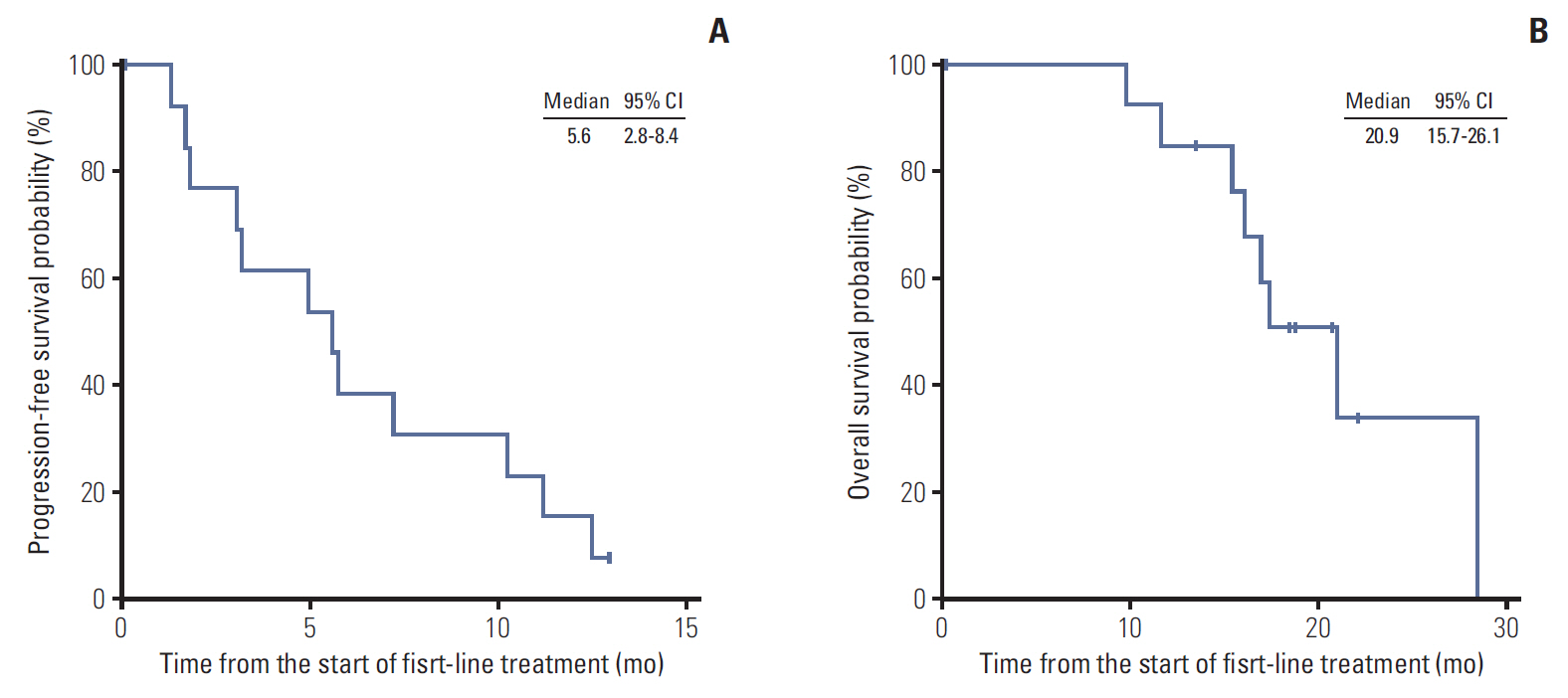
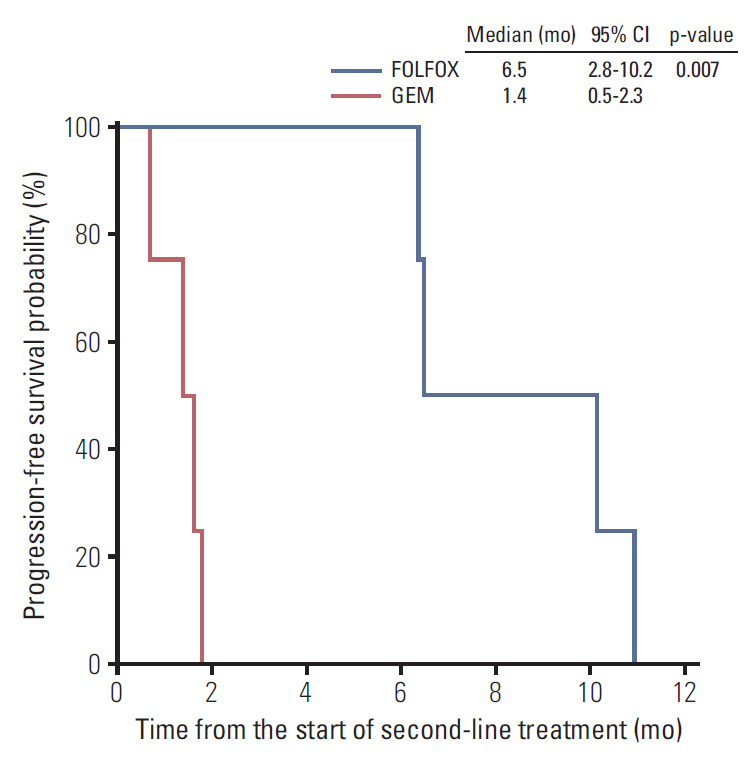
The median age was 58 years. Eleven and four patients had recurrent/metastatic and locally advanced unresectable disease. The median overall survival in all patients was 20.9 months (95% confidence interval [CI], 15.7 to 26.1). As first-line therapy, intravenous 5-fluorouracil were administered in four patients (27%), gemcitabine in five (33%), gemcitabine plus capecitabine in two (13%), oxaliplatin plus 5-fluorouracil/leucovorin (FOLFOX) in two (13%), and concurrent chemoradiotherapy followed by capecitabine maintenance therapy in two (13%). The objective response rate (ORR) to chemotherapy alone was 23% and the median progression-free survival (PFS) was 5.6 months (95% CI, 2.8 to 8.4). After progression, second-line chemotherapy was administered in eight patients, while four patients received FOLFOX and the other four patients received gemcitabine. The ORR was 38%, and patients administered FOLFOX had significantly better PFS than those administered gemcitabine (median, 6.5 months vs. 1.4 months; p=0.007). The ratio of time to tumor progression (TTP) during first-line chemotherapy to TTP at second-line chemotherapy was significantly higher in patients administered FOLFOX (4.07; range, 0.87 to 8.30) than in those administered gemcitabine (0.12; range, 0.08 to 0.25; p=0.029).
CONCLUSION
Our results suggest that oxaliplatin-containing regimens may have improved activity against pancreatic ACC.
MeSH Terms
Figure
Reference
-
References
1. Chen J, Baithun SI. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J Pathol. 1985; 146:17–29.
Article2. Ordonez NG. Pancreatic acinar cell carcinoma. Adv Anat Pathol. 2001; 8:144–59.3. Seth AK, Argani P, Campbell KA, Cameron JL, Pawlik TM, Schulick RD, et al. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg. 2008; 12:1061–7.
Article4. Mortenson MM, Katz MH, Tamm EP, Bhutani MS, Wang H, Evans DB, et al. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008; 196:100–13.
Article5. Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002; 20:4673–8.
Article6. La Rosa S, Adsay V, Albarello L, Asioli S, Casnedi S, Franzi F, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol. 2012; 36:1782–95.7. Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas: a clinicopathologic study of 28 cases. Am J Surg Pathol. 1992; 16:815–37.8. Butturini G, Pisano M, Scarpa A, D'Onofrio M, Auriemma A, Bassi C. Aggressive approach to acinar cell carcinoma of the pancreas: a single-institution experience and a literature review. Langenbecks Arch Surg. 2011; 396:363–9.
Article9. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article10. Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, et al. Outcome of patients with advanced gastrointestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005; 41:1751–7.11. Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008; 144:141–8.
Article12. Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008; 12:2078–86.
Article13. Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007; 35:42–6.
Article14. Lowery MA, Klimstra DS, Shia J, Yu KH, Allen PJ, Brennan MF, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011; 16:1714–20.
Article15. Von Hoff DD. There are no bad anticancer agents, only bad clinical trial designs: twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res. 1998; 4:1079–86.16. Cousin S, Blay JY, Bertucci F, Isambert N, Italiano A, Bompas E, et al. Correlation between overall survival and growth modulation index in pre-treated sarcoma patients: a study from the French Sarcoma Group. Ann Oncol. 2013; 24:2681–5.
Article17. Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002; 160:953–62.18. Furlan D, Sahnane N, Bernasconi B, Frattini M, Tibiletti MG, Molinari F, et al. APC alterations are frequently involved in the pathogenesis of acinar cell carcinoma of the pancreas, mainly through gene loss and promoter hypermethylation. Virchows Arch. 2014; 464:553–64.
Article19. Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014; 4:1398–405.
Article20. Simon M, Bioulac-Sage P, Trillaud H, Blanc JF. FOLFOX regimen in pancreatic acinar cell carcinoma: case report and review of the literature. Acta Oncol. 2012; 51:403–5.
Article21. Pfrommer S, Weber A, Dutkowski P, Schafer NG, Mullhaupt B, Bourquin JP, et al. Successful salvage chemotherapy with FOLFIRINOX for recurrent mixed acinar cell carcinoma and ductal adenocarcinoma of the pancreas in an adolescent patient. Case Rep Oncol. 2013; 6:497–503.
Article22. Ang C, Herran LA, Lagunes DR, Klimstra DS, Kemeny NE. A case report of a patient with advanced acinar cell carcinoma of the pancreas: long-term survival with regional, systemic and targeted therapy. Tumori. 2013; 99:e61.
Article23. Skoulidis F, Cassidy LD, Pisupati V, Jonasson JG, Bjarnason H, Eyfjord JE, et al. Germline Brca2 heterozygosity promotes Kras(G12D)-driven carcinogenesis in a murine model of familial pancreatic cancer. Cancer Cell. 2010; 18:499–509.24. Furukawa T, Sakamoto H, Takeuchi S, Ameri M, Kuboki Y, Yamamoto T, et al. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep. 2015; 5:8829.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polyarthritis and Pancreatic Panniculitis in a Patient with Pancreatic Acinar Cell Carcinoma
- Capecitabine Plus Oxaliplatin Combination Therapy for Basal Cell Carcinoma
- Oxaliplatin-induced Sudden Hearing Loss in a Patient with Pancreatic Cancer
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- Acinar Cell Carcinoma of the Pancreas: A case report