Ann Surg Treat Res.
2017 Jun;92(6):389-395. 10.4174/astr.2017.92.6.389.
A simple rat model of in situ reversible obstructive jaundice in situ reversible obstructive jaundice model
- Affiliations
-
- 1Center for Hepatopancreatobiliary Diseases, Beijing Tsinghua Changgung Hospital, Tsinghua University Medical Center, Beijing, China. dongjiahong@mail.tsinghua.edu.cn
- 2Department and Institute of Hepatobiliary Surgery, Chinese PLA General Hospital, Beijing, China.
- KMID: 2387411
- DOI: http://doi.org/10.4174/astr.2017.92.6.389
Abstract
- PURPOSE
To develop a simple and reliable rat model of in situ reversible obstructive jaundice with low morbidity and mortality rates.
METHODS
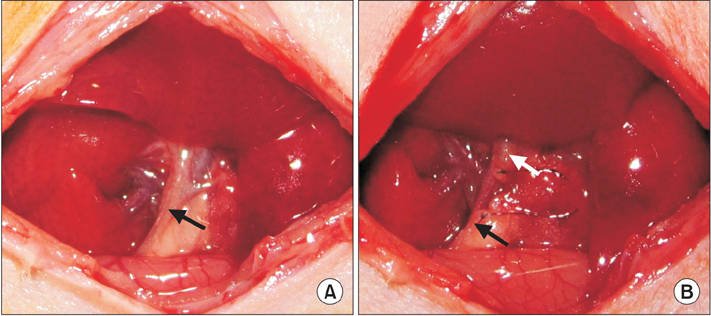
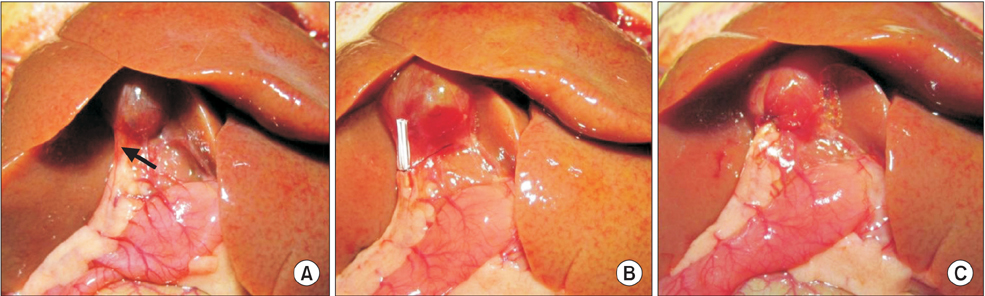
Rats were divided into 4 groups with 8 rats each: the sham-operated (SH) group only underwent laparotomy, the control internal drainage (ID-C) group underwent choledochoduodenostomy, the new internal drainage (ID-N) group and the long-term internal drainage (ID-L) group underwent choledochocholedochostomy. Common bile duct ligation was performed in all the drainage groups 7 days before reversal procedures. All rats were sacrificed for samples 7 days after the last operation except rats of the ID-L group that survived 28 days before sacrifice. Body weight, liver function, histopathological changes, morbidity and mortality were assessed.
RESULTS
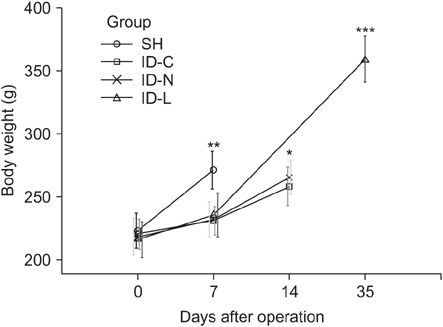
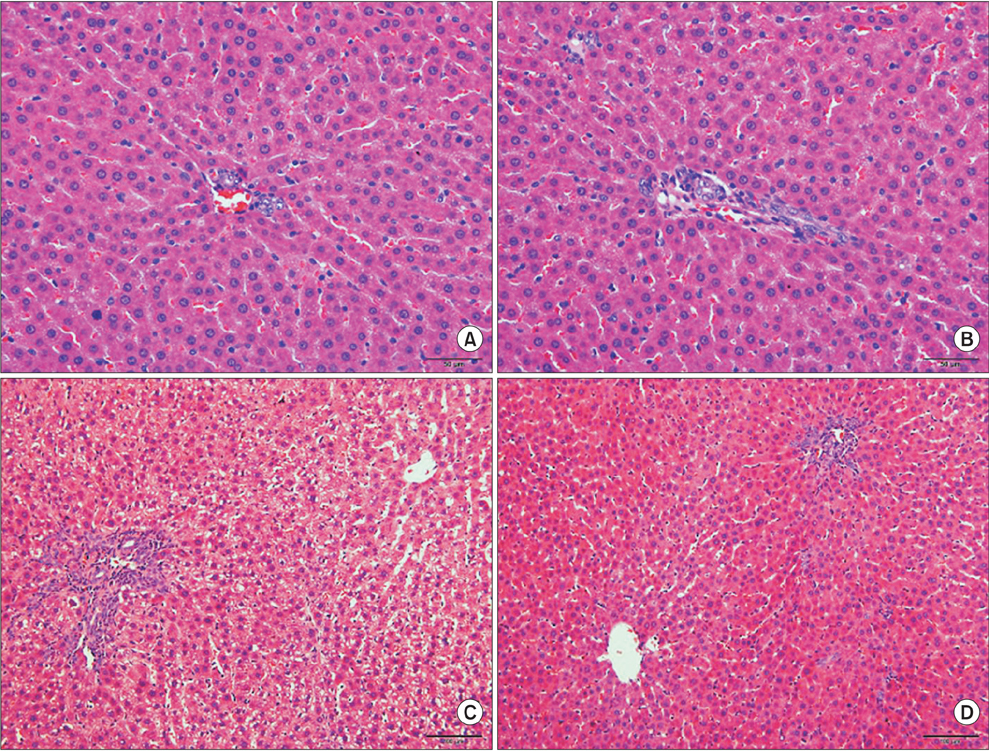
One rat died and 2 rats had complications with tube blockage in the ID-C group. No death or complications occurred in the ID-N and ID-L groups. The drainage tube remained patent in the long-term observation ID-L group. Body weight showed no significant difference between the ID-C and ID-N groups after 7 days drainage. Liver function was not fully recovered in the ID-C and ID-N groups after 7 days drainage, but statistical differences were only observed in the ID-C group compared with the SH and ID-L groups. Periportal inflammation and bile duct proliferation showed severer in the ID-C group than in the ID-N group.
CONCLUSION
The present study provided an efficient, simple, and reliable rat model that is especially suitable for long-term or consecutive studies of reversible obstructive jaundice.
Keyword
MeSH Terms
Figure
Reference
-
1. Wang ZK, Xiao JG, Huang XF, Gong YC, Li W. Effect of biliary drainage on inducible nitric oxide synthase, CD14 and TGR5 expression in obstructive jaundice rats. World J Gastroenterol. 2013; 19:2319–2330.2. Lai EC, Lau SH, Lau WY. The current status of preoperative biliary drainage for patients who receive pancreaticoduodenectomy for periampullary carcinoma: a comprehensive review. Surgeon. 2014; 12:290–296.3. Wang S, Wang X, Li L, Dai H, Han J. Association of preoperative obstructive jaundice with postoperative infectious complications following pancreaticoduodenectomy. Hepatogastroenterology. 2013; 60:1274–1279.4. Mizuguchi K, Ajiki T, Onoyama H, Tomita M, Kuroda Y. Short-term effects of external and internal biliary drainage on liver and cellular immunity in experimental obstructive jaundice. J Hepatobiliary Pancreat Surg. 2004; 11:176–180.5. Li W, Chung SC. An improved rat model of obstructive jaundice and its reversal by internal and external drainage. J Surg Res. 2001; 101:4–15.6. Yu JL, Wang LQ, Andersson R, Persson BG, Bengmark S. New model of reversible obstructive jaundice in rats. Eur J Surg. 1993; 159:163–166.7. Hsien CS, Huang CC, Huang LT, Chung JC, Chou MH. Reversible cholestasis and cholangitis induced by biliary drainage and infusion in the rat. Eur Surg Res. 2006; 38:11–17.8. Fukushima S, Okuno H, Shibatani N, Nakahashi Y, Seki T, Okazaki K. Effect of biliary obstruction and internal biliary drainage on hepatic cytochrome P450 isozymes in rats. World J Gastroenterol. 2008; 14:2556–2560.9. Wu L, Li W, Wang Z, Yuan Z, Hyder Q. Bile acid-induced expression of farnesoid X receptor as the basis for superiority of internal biliary drainage in experimental biliary obstruction. Scand J Gastroenterol. 2013; 48:496–503.10. Wang H, Li C, Xu H, Hu J, Zhang A, Ye S, et al. Precise reconstruction of veins and bile ducts in rat liver transplantation. Cell Biochem Biophys. 2014; 68:55–65.11. Ryan CJ, Than T, Blumgart LH. Choledochoduodenostomy in the rat with obstructive jaundice. J Surg Res. 1977; 23:321–331.12. Hirazawa K, Oka M, Ogura Y, Miyahara M, Hazama S, Suzuki T. New technique for inducing reversible obstructive jaundice in the rat. Eur Surg Res. 1997; 29:195–201.13. Diamond T, Dolan S, Rowlands BJ. An improved technique for choledochoduodenostomy in a rat model of obstructive jaundice. Lab Anim Sci. 1991; 41:82–83.14. Lee JS, Hong TH. The rat choledochojejunostomy model for microsurgical training. Ann Surg Treat Res. 2016; 90:246–249.15. Takagi T, Yokoyama Y, Kokuryo T, Yamaguchi J, Nagino M. Liver regeneration following experimental major hepatectomy with choledochojejunostomy. Br J Surg. 2015; 102:1410–1417.16. Kahramansoy N, Erkol H, Yilmaz EE, Şit M, Yilmaz F, Tosun M, et al. A new model of reversible obstructive jaundice using rapidly absorbable suture materials. Clin Invest Med. 2012; 35:E351–E357.17. Matsumoto T, Ajiki T, Kajiwara E, Mita Y, Fujita T, Morimoto H, et al. Decreased expression of intestinal chemokine TECK/CCL25 in experimental obstructive jaundice and its reversal following internal biliary drainage. J Gastroenterol. 2008; 43:390–396.18. Liu YJ, Mao EQ, Ouyang B, Chen J, Tang YQ, Huang SW, et al. Effect of biliary tract external drainage on cytokine expression and histomorphology of intestine, liver, and lung in rats with hemorrhagic shock. Crit Care Med. 2009; 37:2800–2806.19. Yiming Z, Lei J, Renyou Z, Shan K, Xue L, Min L, et al. Evaluation of the uptake function of liver in rats with obstructive jaundice before and after relief from obstruction by superparamagnetic iron oxide-enhanced magnetic resonance imaging. Oncol Lett. 2012; 4:221–226.20. Padillo FJ, Cruz A, Segura-Jiménez I, Ruiz-Rabelo J, Vázquez-Ezquerra MR, Perea-Alvarez MD, et al. Anti-TNF-alpha treatment and bile duct drainage restore cellular immunity and prevent tissue injury in experimental obstructive jaundice. Int J Immunopathol Pharmacol. 2007; 20:855–860.21. Tian Y, Xia M, Zhang S, Fu Z, Wen Q, Liu F, et al. Initial study of sediment antagonism and characteristics of silver nanoparticle-coated biliary stents in an experimental animal model. Int J Nanomedicine. 2016; 11:1807–1817.22. Oruc MT, Ozmen MM, Han U. A new technique for inducing and releasing obstructive jaundice in rats. Eur Surg Res. 2009; 43:354–359.23. Mizuta A, Chijiiwa K, Saiki S, Kuroki S, Nakamura K, Tanaka M. Differences in biliary lipid excretion after major hepatectomy in obstructive jaundiced rats with preoperative internal, external, or no biliary drainage. Eur Surg Res. 2002; 34:291–299.24. Sano T, Ajiki T, Takeyama Y, Kuroda Y. Internal biliary drainage improves decreased number of gut mucosal T lymphocytes and MAdCAM-1 expression in jaundiced rats. Surgery. 2004; 136:693–699.25. Suzuki H, Iyomasa S, Nimura Y, Yoshida S. Internal biliary drainage, unlike external drainage, does not suppress the regeneration of cholestatic rat liver after partial hepatectomy. Hepatology. 1994; 20:1318–1322.