Yonsei Med J.
2014 Mar;55(2):283-291.
Cytotoxic Potential of Silver Nanoparticles
- Affiliations
-
- 1Laboratory of Plasma Physics & Materials, Beijing Institute of Graphic Communication, Beijing, China. lppmchenqiang@hotmail.com
- 2CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, National Center for Nanoscience and Technology, Beijing, China. chenchy@nanoctr.cn
Abstract
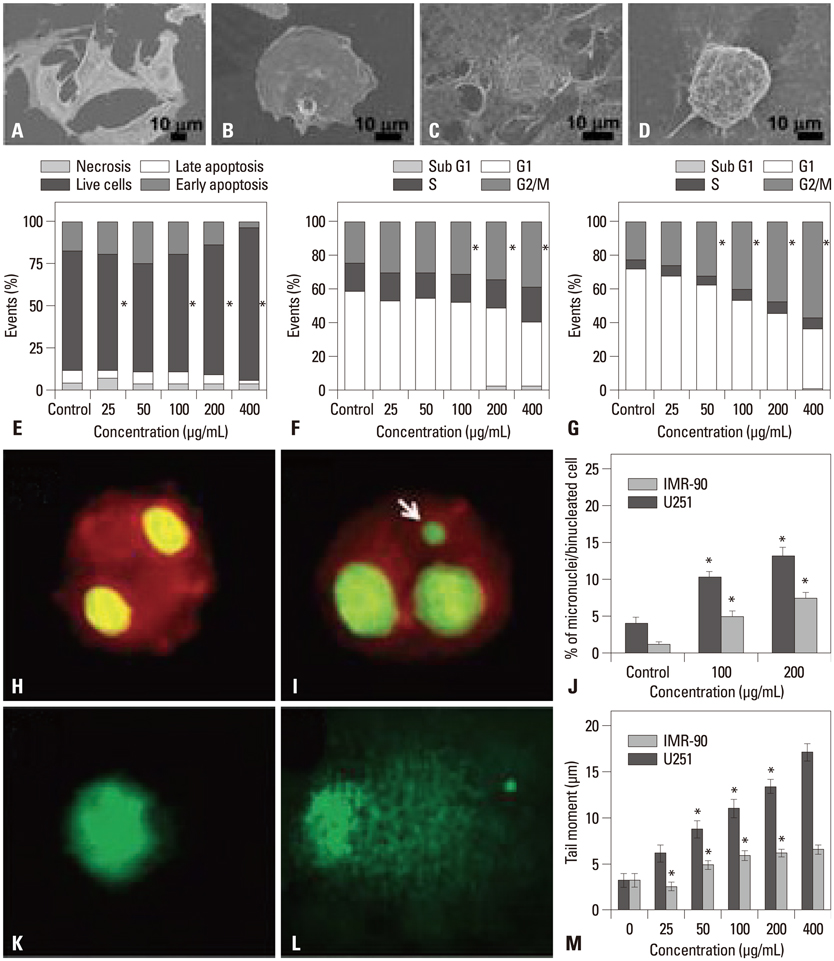
- Silver nanoparticles (AgNPs) have been widely used in industrial, household, and healthcare-related products due to their excellent antimicrobial activity. With increased exposure of AgNPs to human beings, the risk of safety has attracted much attention from the public and scientists. In review of recent studies, we discuss the potential impact of AgNPs on individuals at the cell level. In detail, we highlight the main effects mediated by AgNPs on the cell, such as cell uptake and intracellular distribution, cytotoxicity, genotoxicity, and immunological responses, as well as some of the major factors that influence these effects in vivo and in vivo, such as dose, time, size, shape, surface chemistry, and cell type. At the end, we summarize the main influences on the cell and indicate the challenges in this field, which may be helpful for assessing the risk of AgNPs in future.
Keyword
Figure
Reference
-
1. Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013; 52:1636–1653.
Article2. Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008; 176:1–12.
Article3. He W, Zhou YT, Wamer WG, Boudreau MD, Yin JJ. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials. 2012; 33:7547–7555.
Article4. Dickerson MB, Sandhage KH, Naik RR. Protein- and peptide-directed syntheses of inorganic materials. Chem Rev. 2008; 108:4935–4978.
Article5. Eckhardt S, Brunetto PS, Gagnon J, Priebe M, Giese B, Fromm KM. Nanobio silver: its interactions with peptides and bacteria, and its uses in medicine. Chem Rev. 2013; 113:4708–4754.
Article6. Tejamaya M, Römer I, Merrifield RC, Lead JR. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol. 2012; 46:7011–7017.
Article7. Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009; 190:156–162.
Article8. Ahamed M, Alsalhi MS, Siddiqui MK. Silver nanoparticle applications and human health. Clin Chim Acta. 2010; 411:1841–1848.
Article9. Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW, et al. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials. 2010; 31:8350–8361.
Article10. Hyun JS, Lee BS, Ryu HY, Sung JH, Chung KH, Yu IJ. Effects of repeated silver nanoparticles exposure on the histological structure and mucins of nasal respiratory mucosa in rats. Toxicol Lett. 2008; 182:24–28.
Article11. Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004; 16:437–445.
Article12. Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009; 187:15–21.
Article13. Lee HY, Choi YJ, Jung EJ, Yin HQ, Kwon JT, Kim JE, et al. Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation. J Nanopart Res. 2009; 12:1567–1578.
Article14. Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008; 20:575–583.
Article15. Cha K, Hong HW, Choi YG, Lee MJ, Park JH, Chae HK, et al. Comparison of acute responses of mice livers to short-term exposure to nano-sized or micro-sized silver particles. Biotechnol Lett. 2008; 30:1893–1899.
Article16. Song KS, Sung JH, Ji JH, Lee JH, Lee JS, Ryu HR, et al. Recovery from silver-nanoparticle-exposure-induced lung inflammation and lung function changes in Sprague Dawley rats. Nanotoxicology. 2013; 7:169–180.
Article17. Scown TM, Santos EM, Johnston BD, Gaiser B, Baalousha M, Mitov S, et al. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci. 2010; 115:521–534.
Article18. Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007; 1:133–143.
Article19. Wu Y, Zhou Q, Li H, Liu W, Wang T, Jiang G. Effects of silver nanoparticles on the development and histopathology biomarkers of Japanese medaka (Oryzias latipes) using the partial-life test. Aquat Toxicol. 2010; 100:160–167.
Article20. Lee JH, Mun J, Park JD, Yu IJ. A health surveillance case study on workers who manufacture silver nanomaterials. Nanotoxicology. 2012; 6:667–669.
Article21. Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R. Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology. 2011; 22:375101.
Article22. Asharani PV, Hande MP, Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009; 10:65.
Article23. Singh RP, Ramarao P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol Lett. 2012; 213:249–259.
Article24. Wang H, Wu L, Reinhard BM. Scavenger receptor mediated endocytosis of silver nanoparticles into J774A.1 macrophages is heterogeneous. ACS Nano. 2012; 6:7122–7132.
Article25. AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009; 3:279–290.
Article26. Yang EJ, Kim S, Kim JS, Choi IH. Inflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012; 33:6858–6867.
Article27. Jiang X, Foldbjerg R, Miclaus T, Wang L, Singh R, Hayashi Y, et al. Multi-platform genotoxicity analysis of silver nanoparticles in the model cell line CHO-K1. Toxicol Lett. 2013; 222:55–63.
Article28. Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y. Silver ion induces a cyclosporine a-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem. 2003; 134:43–49.
Article29. Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol Lett. 2008; 179:93–100.
Article30. Kim YJ, Yang SI, Ryu JC. Cytotoxicity and genotoxicity of nano-silver in mammalian cell lines. Mol Cell Toxicol. 2010; 6:119–125.
Article31. Kawata K, Osawa M, Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 2009; 43:6046–6051.
Article32. Jang J, Lim DH, Choi IH. The impact of nanomaterials in immune system. Immune Netw. 2010; 10:85–91.
Article33. Trickler WJ, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, et al. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci. 2010; 118:160–170.
Article34. Park J, Lim DH, Lim HJ, Kwon T, Choi JS, Jeong S, et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun (Camb). 2011; 47:4382–4384.
Article35. Li L, Sun J, Li X, Zhang Y, Wang Z, Wang C, et al. Controllable synthesis of monodispersed silver nanoparticles as standards for quantitative assessment of their cytotoxicity. Biomaterials. 2012; 33:1714–1721.
Article36. Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, et al. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology. 2010; 4:319–330.
Article37. Kittler S, Greulich C, Diendorf J, Köller M, Epple M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater. 2010; 22:4548–4554.
Article38. He D, Bligh MW, Waite TD. Effects of aggregate structure on the dissolution kinetics of citrate-stabilized silver nanoparticles. Environ Sci Technol. 2013; 47:9148–9156.
Article39. Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008; 3:145–150.
Article40. Lu W, Senapati D, Wang S, Tovmachenko O, Singh AK, Yu H, et al. Effect of Surface Coating on the Toxicity of Silver Nanomaterials on Human Skin Keratinocytes. Chem Phys Lett. 2010; 487:92–96.
Article41. Kittler S, Greulich C, Gebauer JS, Diendorf J, Treuel L, Ruiz L. The influence of proteins on the dispersability and cell-biological activity of silver nanoparticles. J Mater Chem. 2010; 20:512–518.
Article42. Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol. 2008; 233:404–410.
Article43. Stoehr LC, Gonzalez E, Stampfl A, Casals E, Duschl A, Puntes V, et al. Shape matters: effects of silver nanospheres and wires on human alveolar epithelial cells. Part Fibre Toxicol. 2011; 8:36.
Article44. Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008; 179:130–139.
Article45. Wang L, Liu Y, Li W, Jiang X, Ji Y, Wu X, et al. Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano Lett. 2011; 11:772–780.
Article46. Zhang L, Wang L, Hu Y, Liu Z, Tian Y, Wu X, et al. Selective metabolic effects of gold nanorods on normal and cancer cells and their application in anticancer drug screening. Biomaterials. 2013; 34:7117–7126.
Article47. Choi J, Reipa V, Hitchins VM, Goering PL, Malinauskas RA. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol Sci. 2011; 123:133–143.
Article48. Qu Y, Li W, Zhou Y, Liu X, Zhang L, Wang L, et al. Full assessment of fate and physiological behavior of quantum dots utilizing Caenorhabditis elegans as a model organism. Nano Lett. 2011; 11:3174–3183.
Article49. Li YF, Chen C. Fate and toxicity of metallic and metal-containing nanoparticles for biomedical applications. Small. 2011; 7:2965–2980.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Silver Nanoparticles as a Smart Antimicrobial Agent
- Phagocytosis and Endocytosis of Silver Nanoparticles Induce Interleukin-8 Production in Human Macrophages
- Inhibition Effects of Silver Nanoparticles against Powdery Mildews on Cucumber and Pumpkin
- Application of Silver Nanoparticles for the Control of Colletotrichum Species In Vitro and Pepper Anthracnose Disease in Field
- A Transfer of Silver Nanoparticles from Pregnant Rat to Offspring