J Korean Med Sci.
2017 Sep;32(9):1502-1507. 10.3346/jkms.2017.32.9.1502.
The Anti-Inflammatory Effects of Oral-Formulated Tacrolimus in Mice with Experimental Autoimmune Encephalomyelitis
- Affiliations
-
- 1Department of Neurology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea. icandr@hanmail.net
- 2Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Neurology, Hanyang University Hospital, Hanyang University College of Medicine, Seoul, Korea.
- 4Department of Otorhinolaryngology, Head and Neck Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2385958
- DOI: http://doi.org/10.3346/jkms.2017.32.9.1502
Abstract
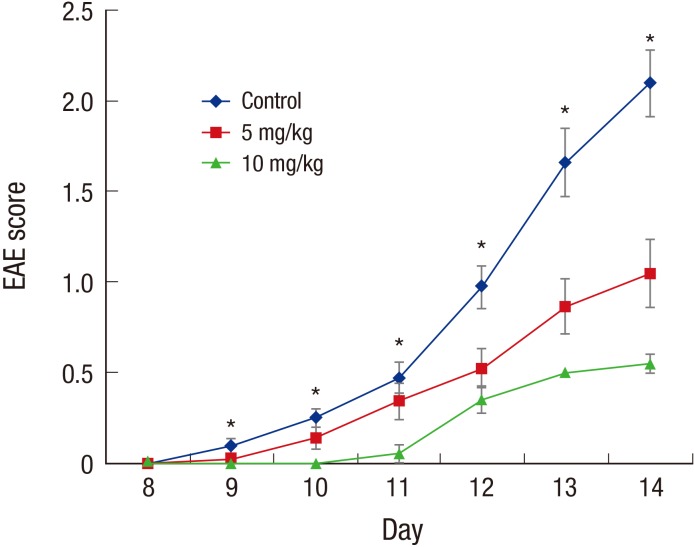
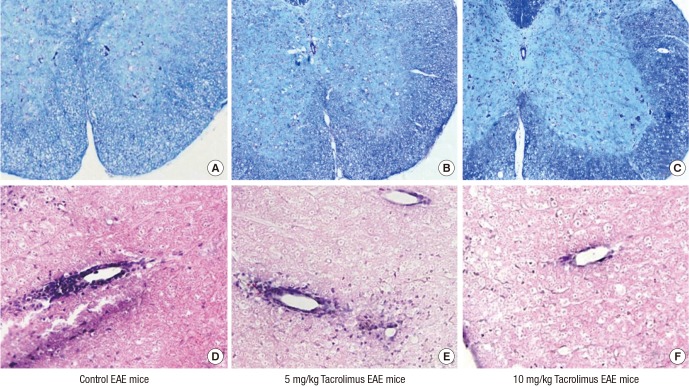
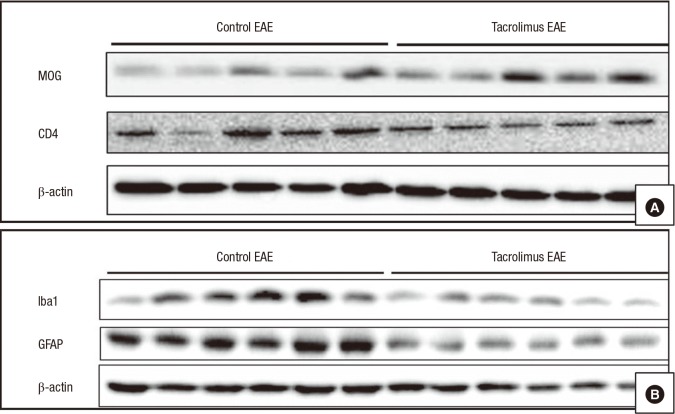
- Multiple sclerosis (MS) is a T-lymphocyte-mediated autoimmune disease that is characterized by inflammation in the central nervous system (CNS). Although many disease-modifying therapies (DMTs) are presumed effective in patients with MS, studies on the efficacy and safety of DMTs for preventing MS relapse are limited. Therefore, we tested the immunosuppressive anti-inflammatory effects of oral-formulated tacrolimus (FK506) on MS in a mouse model of experimental autoimmune encephalomyelitis (EAE). The mice were randomly divided into 3 experimental groups: an untreated EAE group, a low-dose tacrolimus-treated EAE group, and a high-dose tacrolimus-treated EAE group. After autoimmunization of the EAE mice with myelin oligodendrocyte glycoprotein, symptom severity scores, immunohistochemistry of the myelination of the spinal cord, and western blotting were used to evaluate the EAE mice. After the autoimmunization, the symptom scores of each EAE group significantly differed at times. The group treated with the larger tacrolimus dose had the lowest symptom scores. The tacrolimus-treated EAE groups exhibited less demyelination and inflammation and weak immunoreactivity for all of the immunization biomarkers. Our results revealed that oral-formulated tacrolimus inhibited the autoimmunization in MS pathogenesis by inactivating inflammatory cells.
Keyword
MeSH Terms
-
Animals
Autoimmune Diseases
Biomarkers
Blotting, Western
Central Nervous System
Demyelinating Diseases
Encephalomyelitis, Autoimmune, Experimental*
Humans
Immunization
Immunohistochemistry
Inflammation
Mice*
Multiple Sclerosis
Myelin Sheath
Myelin-Oligodendrocyte Glycoprotein
Neuromyelitis Optica
Recurrence
Spinal Cord
Tacrolimus*
Biomarkers
Myelin-Oligodendrocyte Glycoprotein
Tacrolimus
Figure
Cited by 1 articles
-
The Anti-Inflammatory Effect of Sulforaphane in Mice with Experimental Autoimmune Encephalomyelitis
Il-Han Yoo, Myung-Jin Kim, Jiyoung Kim, Jung-Joon Sung, Sung Taek Park, Suk-Won Ahn
J Korean Med Sci. 2019;34(28):. doi: 10.3346/jkms.2019.34.e197.
Reference
-
1. Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372:1502–1517. PMID: 18970977.2. Bar-Or A. Immunology of multiple sclerosis. Neurol Clin. 2005; 23:149–175. PMID: 15661092.3. Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006; 354:942–955. PMID: 16510748.4. Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989; 112:133–146. PMID: 2917275.5. Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010; 9:883–897. PMID: 21031003.6. Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Bauer L, Jakobs P, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006; 67:1242–1249. PMID: 16914693.7. Neuhaus O, Farina C, Wekerle H, Hohlfeld R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001; 56:702–708. PMID: 11288751.8. Mikol DD, Barkhof F, Chang P, Coyle PK, Jeffery DR, Schwid SR, Stubinski B, Uitdehaag B; REGARD study group. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008; 7:903–914. PMID: 18789766.9. Walther EU, Hohlfeld R. Multiple sclerosis: side effects of interferon beta therapy and their management. Neurology. 1999; 53:1622–1627. PMID: 10563602.10. Herndon RM, Rudick RA, Munschauer FE 3rd, Mass MK, Salazar AM, Coats ME, Labutta R, Richert JR, Cohan SL, Genain C, et al. Eight-year immunogenicity and safety of interferon beta-1a-Avonex treatment in patients with multiple sclerosis. Mult Scler. 2005; 11:409–419. PMID: 16042223.11. Pahan K. Neuroimmune pharmacological control of EAE. J Neuroimmune Pharmacol. 2010; 5:165–167. PMID: 20414732.12. Furukawa Y, Yoshikawa H, Iwasa K, Yamada M. Clinical efficacy and cytokine network-modulating effects of tacrolimus in myasthenia gravis. J Neuroimmunol. 2008; 195:108–115. PMID: 18262659.13. Friedrich RB, Coradini K, Fonseca FN, Guterres SS, Beck RC, Pohlmann AR. Lipid-core nanocapsules improved antiedematogenic activity of tacrolimus in adjuvant-induced arthritis model. J Nanosci Nanotechnol. 2016; 16:1265–1274. PMID: 27433576.14. Mondal S, Roy A, Pahan K. Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis. J Immunol. 2009; 182:5013–5023. PMID: 19342681.15. Kuerten S, Lehmann PV. The immune pathogenesis of experimental autoimmune encephalomyelitis: lessons learned for multiple sclerosis? J Interferon Cytokine Res. 2011; 31:907–916. PMID: 21936633.16. Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2010; Chapter 15:Unit 15.1.17. Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995; 25:1951–1959. PMID: 7621871.18. Pachner AR. Experimental models of multiple sclerosis. Curr Opin Neurol. 2011; 24:291–299. PMID: 21519255.19. van der Star BJ, Vogel DY, Kipp M, Puentes F, Baker D, Amor S. In vitro and in vivo models of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012; 11:570–588. PMID: 22583443.20. Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J Immunol. 2002; 169:117–125. PMID: 12077236.21. Cinar O, Semiz O, Can A. A microscopic survey on the efficiency of well-known routine chemical fixatives on cryosections. Acta Histochem. 2006; 108:487–496. PMID: 16950501.22. Lamberts R, Goldsmith PC. Fixation, fine structure, and immunostaining for neuropeptides: perfusion versus immersion of the neuroendocrine hypothalamus. J Histochem Cytochem. 1986; 34:389–398. PMID: 2419392.23. Mondal S, Pahan K. Cinnamon ameliorates experimental allergic encephalomyelitis in mice via regulatory T cells: implications for multiple sclerosis therapy. PLoS One. 2015; 10:e0116566. PMID: 25569428.24. Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004; 199:971–979. PMID: 15067033.25. Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998; 338:278–285. PMID: 9445407.26. Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006; 66:1696–1702. PMID: 16769943.27. Petersen B, Bendtzen K, Koch-Henriksen N, Ravnborg M, Ross C, Sorensen PS; Danish Multiple Sclerosis Group. Persistence of neutralizing antibodies after discontinuation of IFNbeta therapy in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2006; 12:247–252. PMID: 16764336.28. Frohman EM, Brannon K, Alexander S, Sims D, Phillips JT, O’Leary S, Hawker K, Racke MK. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004; 10:302–307. PMID: 15222696.29. Gold BG, Voda J, Yu X, McKeon G, Bourdette DN. FK506 and a nonimmunosuppressant derivative reduce axonal and myelin damage in experimental autoimmune encephalomyelitis: neuroimmunophilin ligand-mediated neuroprotection in a model of multiple sclerosis. J Neurosci Res. 2004; 77:367–377. PMID: 15248293.30. Kang Y, Sun Y, Zhang J, Gao W, Kang J, Wang Y, Wang B, Xia G. Treg cell resistance to apoptosis in DNA vaccination for experimental autoimmune encephalomyelitis treatment. PLoS One. 2012; 7:e49994. PMID: 23166807.31. Jacques F, Gaboury I, Christie S, Grand'maison F. Combination therapy of interferon Beta-1b and tacrolimus: a pilot safety study. Mult Scler Int. 2012; 2012:935921. PMID: 22966460.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glatiramer acetate inhibits the activation of NFkappaB in the CNS of experimental autoimmune encephalomyelitis
- The Anti-Inflammatory Effect of Sulforaphane in Mice with Experimental Autoimmune Encephalomyelitis
- Immunohistochemical Localization of Bcl-2 in the Spinal Cords of Rats with Experimental Autoimmune Encephalomyelitis
- Therapeutic Efficacy of Mesenchymal Stem Cells and Mesenchymal Stem Cells-derived Neural Progenitors in Experimental Autoimmune Encephalomyelitis
- Erythropoietin and autoimmune neuroinflammation: lessons from experimental autoimmune encephalomyelitis and experimental autoimmune neuritis