Korean Circ J.
2017 May;47(3):401-408. 10.4070/kcj.2016.0214.
Effect of Rosuvastatin on Bovine Pericardial Aortic Tissue Valve Calcification in a Rat Subdermal Implantation Model
- Affiliations
-
- 1Division of Thoracic and Cardiovascular Surgery, Severance Cardiovascular Hospital, Yonsei Cardiovascular Research Institute, Yonsei University College of Medicine, Seoul, Korea. SAK911@yuhs.ac
- KMID: 2385395
- DOI: http://doi.org/10.4070/kcj.2016.0214
Abstract
- BACKGROUND AND OBJECTIVES
There are pathophysiologic similarities between calcification and atherosclerosis because both are the product of an active inflammatory process. The aim of this study was to examine the effects of statin treatment on calcification in bovine pericardial tissue valves.
MATERIALS AND METHODS
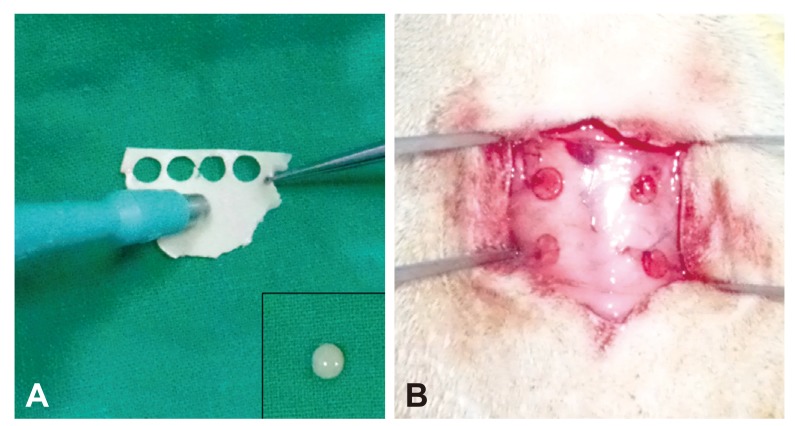
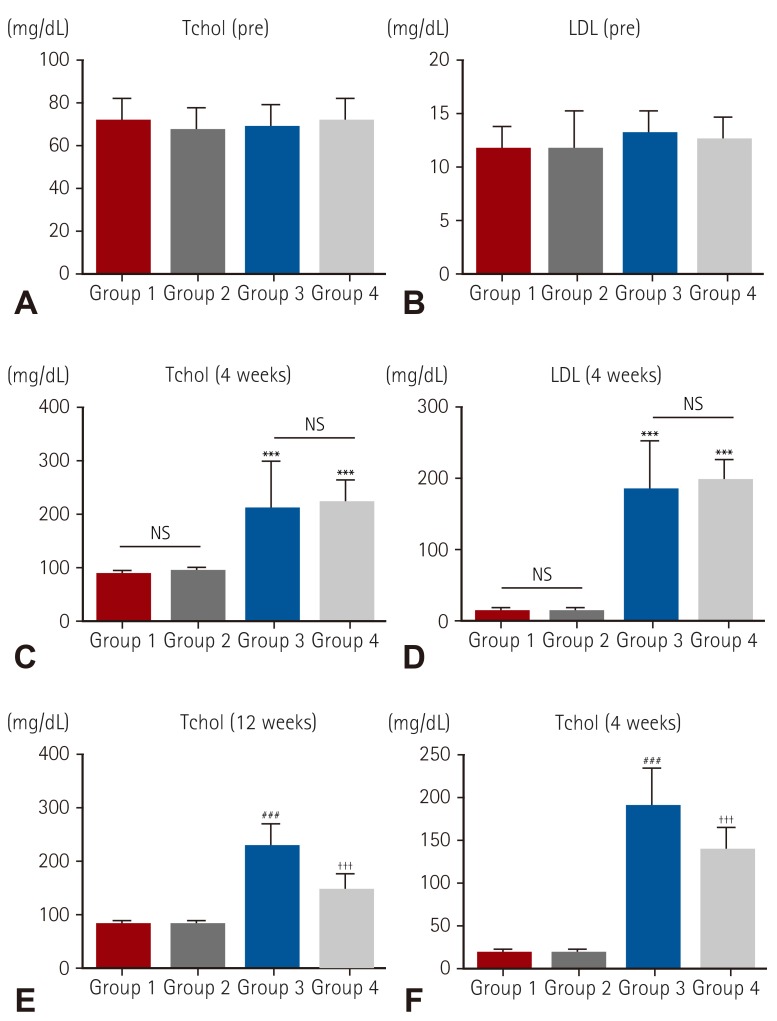
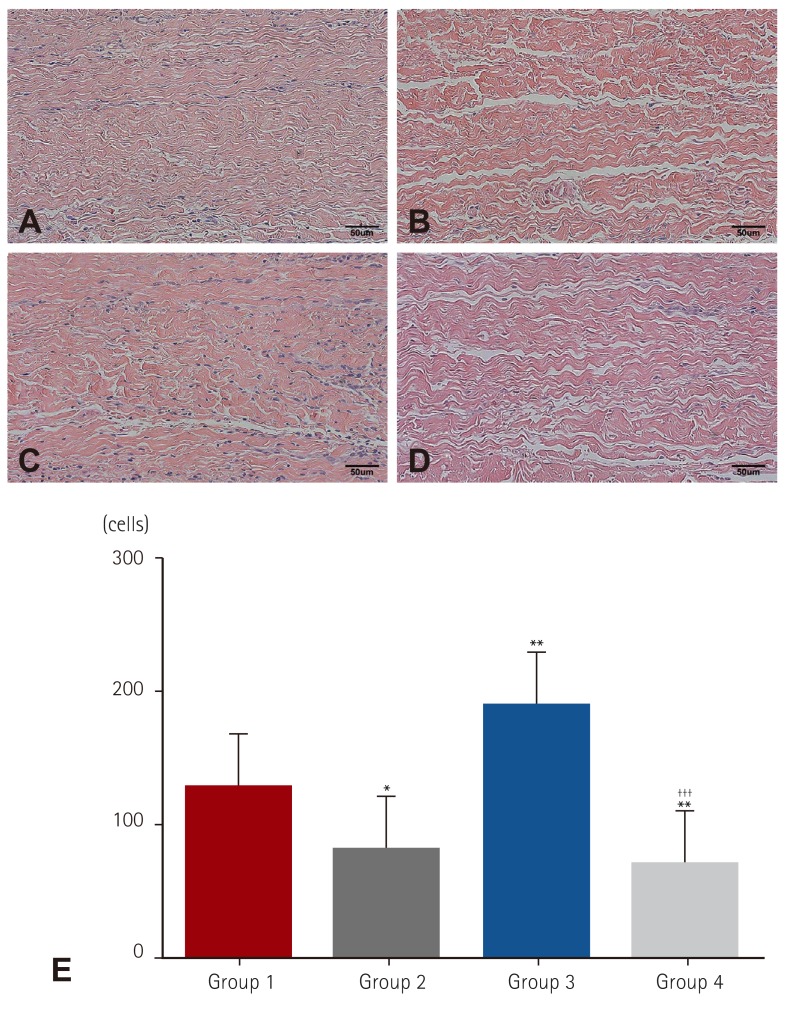
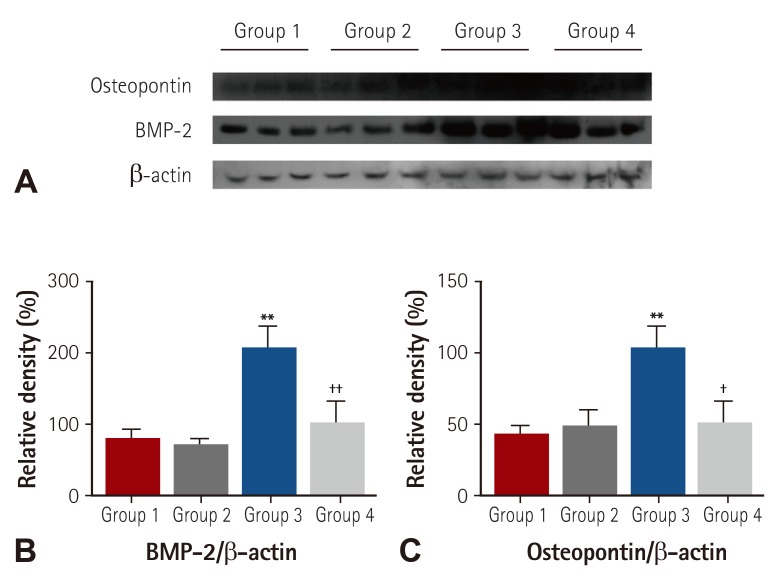
Forty Sprague-Dawley rats were randomly divided into 4 groups according to hypercholesterolemia induction and statin intake (Group 1, n=10: normal diet without statin treatment, Group 2, n=10: normal diet with statin treatment, Group 3, n=10: high fat diet without statin treatment, Group 4, n=10: high fat diet with statin treatment). Serum lipid levels were measured just before the experiment and after 4 and 12 weeks. Bovine pericardial tissue valve cusps were surgically implanted in rat dorsal subcutis at 4 weeks. After the surgery, statin was administered daily to Groups 2 and 4. Serum interleukin-6 (IL-6) level was measured at 5 weeks. Cusps were explanted at 12 weeks and calcium levels were determined by atomic absorption spectroscopy.
RESULTS
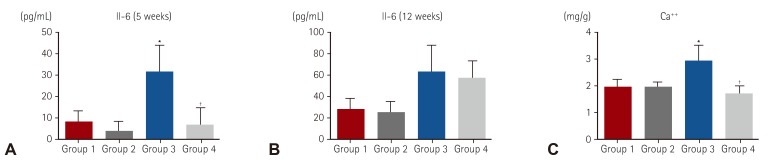
Mean IL-6 was significantly higher in Group 3 at 5 weeks (7.14, 2.03, 31.70, and 6.90 pg/dL for each group, respectively). Mean calcium level in Group 3 was significantly higher among groups but Group 4 was significantly lower compared to Group 3 and was similar to Group 1, 2 (1.86, 1.92, 2.55, and 1.80 mg/g for each group, respectively, p<0.01).
CONCLUSION
Hypercholesterolemia may be a significant risk factor for bovine pericardial valve calcification. Statin treatment significantly attenuated calcification of bovine pericardial valve tissue in a rat subdermal implantation model and might prolong the durability of bioprostheses.
Keyword
MeSH Terms
Figure
Reference
-
1. Cohn LH, Collins JJ Jr, Rizzo RJ, Adams DH, Couper GS, Aranki SF. Twenty-year follow-up of the Hancock modified orifice porcine aortic valve. Ann Thorac Surg. 1998; 66(6 Suppl):S30–S34. PMID: 9930412.2. Jamieson WR, Burr LH, Munro AI, Miyagishima RT. Carpentier-Edwards standard porcine bioprosthesis: a 21-year experience. Ann Thorac Surg. 1998; 66(6 Suppl):S40–S43. PMID: 9930414.3. Schoen FJ. Cardiac valve prostheses: review of clinical status and contemporary biomaterials issues. J Biomed Mater Res. 1987; 21(A1 Suppl):91–117. PMID: 3553196.4. Colli A, Gherli T, Mestres CA, Pomar JL. Degeneration of native and tissue prosthetic valve in aortic position: do statins play an effective role in prevention? Int J Cardiol. 2007; 116:144–152. PMID: 16828903.5. Farivar RS, Cohn LH. Hypercholesterolemia is a risk factor for bioprosthetic valve calcification and explantation. J Thorac Cardiovasc Surg. 2003; 126:969–975. PMID: 14566234.6. Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005; 79:1072–1080. PMID: 15734452.7. Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001; 104:2205–2209. PMID: 11684632.8. Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001; 88:693–695. PMID: 11564402.9. Achenbach S, Ropers D, Pohle K, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002; 106:1077–1082. PMID: 12196332.10. Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. 2000; 101:2497–2502. PMID: 10831524.11. Black AE, Sinz MW, Hayes RN, Woolf TF. Metabolism and excretion studies in mouse after single and multiple oral doses of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab Dispos. 1998; 26:755–763. PMID: 9698289.12. Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002; 40:1723–1730. PMID: 12446053.13. Shavelle DM, Takasu J, Budoff MJ, Mao S, Zhao XQ, O'Brien KD. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet. 2002; 359:1125–1126. PMID: 11943265.14. Lorusso R, Corradi D, Maestri R, et al. Atorvastatin attenuates post-implant tissue degeneration of cardiac prosthetic valve bovine pericardial tissue in a subcutaneous animal model. Int J Cardiol. 2010; 141:68–74. PMID: 19167110.15. Demer LL. Cholesterol in vascular and valvular calcification. Circulation. 2001; 104:1881–1883. PMID: 11602487.16. Vyavahare NR, Jones PL, Hirsch D, Schoen FJ, Levy RJ. Prevention of glutaraldehyde-fixed bioprosthetic heart valve calcification by alcohol pretreatment: further mechanistic studies. J Heart Valve Dis. 2000; 9:561–566. PMID: 10947050.17. Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999; 19:1218–1222. PMID: 10323772.18. Schmermund A, Möhlenkamp S, Erbel R. The latest on the calcium story. Am J Cardiol. 2002; 90(10C):12L–14L.19. Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001; 103:1522–1528. PMID: 11257079.20. Yildirir A, Müderrisoglu H. Non-lipid effects of statins: emerging new indications. Curr Vasc Pharmacol. 2004; 2:309–318. PMID: 15320810.21. Rajamannan NM, Subramaniam M, Springett M, et al. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002; 105:2660–2665. PMID: 12045173.22. Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999; 286:1946–1949. PMID: 10583956.23. Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006; 114(1 Suppl):I547–I552. PMID: 16820635.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement of Porcine Aortic and Pulmonary Valve Geometry and Design for Implantable Tissue Valve
- Entelon150® (Vitis vinifera Seed Extract) Attenuates Degenerative Changes in Intravascular Valve Prostheses in Rabbits
- Neo-Leaflet Failure after Comprehensive Aortic Root and Valve Reconstruction
- Calcium Mitigation in the Bovine Pericardial Tissue in the Rat Subcutaneous Implantation model-MgCl2 Effect
- Anticalcification Treatment of Glutaraldehyde-fixed Bovine Pericardium with Amino Acids (The Effect of Ethanol, Glutamic Acid and Homocysteic Acid Treatment)