Kosin Med J.
2017 Jun;32(1):72-83. 10.7180/kmj.2017.32.1.72.
Utility of Regular Radiological Follow-up on Early Detection of Contralateral Malignancy and Long-term Outcomes in Metachronous Bilateral Breast Cancer Patients
- Affiliations
-
- 1Department of Surgery, Keimyung University School of Medicine, Daegu, Korea. shkang9002@gmail.com
- KMID: 2384835
- DOI: http://doi.org/10.7180/kmj.2017.32.1.72
Abstract
OBJECTIVES
We investigated the utility of regular radiological follow-up on the early detection of contralateral breast cancer(CBC) and prognosis in patients with metachronous bilateral breast cancer.
METHODS
Between 1983 and 2010, 49(2.1%) metachronous bilateral breast cancer patients were identified among a total of 2,343 cases of invasive or in situ breast carcinomas. We reviewed the patients' medical records including age, stage, duration between the first and second breast cancer diagnosis, operation method, recurrence, and breast cancer-specific survival.
RESULTS
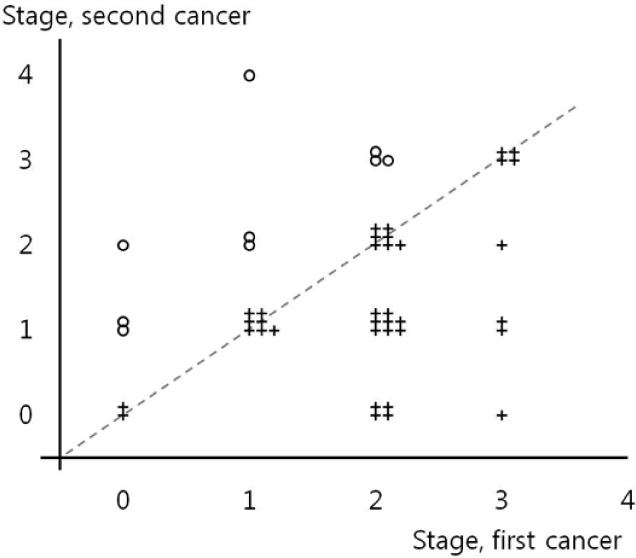
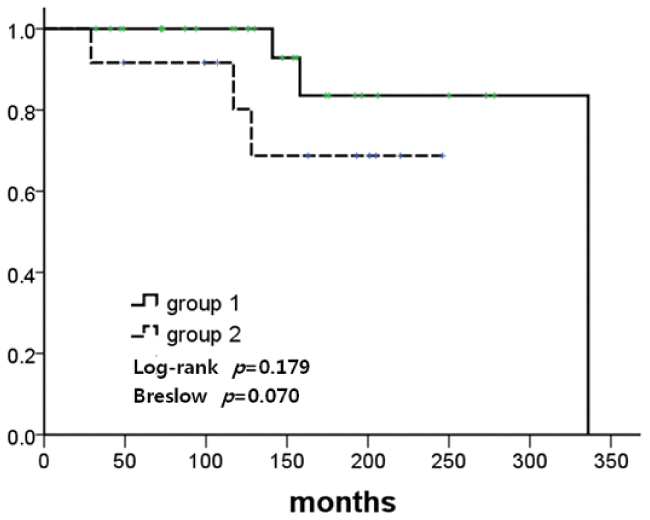
The mean ages at the first and second breast cancer diagnosis were 43.8 and 49.2 years, respectively. The mean duration between the first and second breast cancer diagnosis was 68.9 months (range, 7-266 months). Regular radiological follow-up with annual mammography(MMG) with or without ultrasonography was conducted in 28 patients (63.6%, Group 1), and no regular follow-up was performed in 12 patients (27.3%, Group 2). The median follow-up duration was 150 months. In a comparative analysis, Group 1 patients exhibited more stage 0 and stage 1 malignancies (82.1% vs. 25%, P =0.006) as second cancer and the same or an improved stage (71.4% vs. 33.3%, P =0.042) of second cancer compared to Group 2 patients. Breast cancer-specific survival rates between the two groups after the first cancer occurrence were higher in Group 1 patients compared to Group 2 patients, although this did not reach statistical significance.
CONCLUSION
Screening for CBC with regular radiological follow-up could result in early detection of CBC, less invasive surgical procedures, and enhanced breast cancer-specific survival outcomes.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim Z, Min SY, Yoon CS, Jung KW, Ko BS, Kang E, et al. The basic facts of Korean breast cancer in 2012: results from a nationwide survey and breast cancer registry database. J Breast Cancer. 2015; 18:103–111.
Article2. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. JAMA. 1994; 271:1587–1592.3. Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1994; 271:1593–1597.
Article4. Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013; 31:961–965.
Article5. Lash TL, Fox MP, Buist DS, Wei F, Field TS, Frost FJ, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007; 25:3001–3006.
Article6. Schootman M, Jeffe DB, Lian M, Aft R, Gillanders WE. Surveillance mammography and the risk of death among elderly breast cancer patients. Breast Cancer Res Treat. 2008; 111:489–496.
Article7. Grunfeld E, Noorani H, McGahan L, Paszat L, Coyle D, van Walraven C, et al. Surveillance mammography after treatment of primary breast cancer: a systematic review. Breast. 2002; 11:228–235.
Article8. Houssami N, Abraham LA, Miglioretti DL, Sickles EA, Kerlikowske K, Buist DS, et al. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA. 2011; 305:790–799.
Article9. Houssami N, Ciatto S. Mammographic surveillance in women with a personal history of breast cancer: how accurate? How effective? Breast. 2010; 19:439–445.
Article10. Nichols HB, Berrington de, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011; 29:1564–1569.
Article11. Hartman M, Czene K, Reilly M, Adolfsson J, Bergh J, Adami HO, et al. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol. 2007; 25:4210–4216.
Article12. Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011; 378:771–784.
Article13. Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010; 11:1135–1141.
Article14. Lee Y, Kang E, Lee AS, Baek H, Kim EK, Park SY, et al. Outcomes and recurrence patterns according to breast cancer subtypes in Korean women. Breast Cancer Res Treat. 2015; 151:183–190.
Article15. Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010; 28:2404–2410.
Article16. Pierce LJ, Levin AM, Rebbeck TR, Ben-David MA, Friedman E, Solin LJ, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006; 24:2437–2443.
Article17. Vichapat V, Garmo H, Holmberg L, Fentiman IS, Tutt A, Gillett C, et al. Prognosis of metachronous contralateral breast cancer: importance of stage, age and interval time between the two diagnoses. Breast Cancer Res Treat. 2011; 130:609–618.
Article18. Alkner S, Bendahl PO, Fernö M, Manjer J, Rydén L. Prediction of outcome after diagnosis of metachronous contralateral breast cancer. BMC Cancer. 2011; 11:114.
Article19. Font-Gonzalez A, Liu L, Voogd AC, Schmidt MK, Roukema JA, Coebergh JW, et al. Inferior survival for young patients with contralateral compared to unilateral breast cancer: a nationwide population-based study in the Netherlands. Breast Cancer Res Treat. 2013; 139:811–819.
Article20. Liederbach E, Piro R, Hughes K, Watkin R, Wang CH, Yao K. Clinicopathologic features and time interval analysis of contralateral breast cancers. Surgery. 2015; 158:676–685.
Article21. Portschy PR, Abbott AM, Burke EE, Nzara R, Marmor S, Kuntz KM, et al. Perceptions of contralateral breast cancer risk: a prospective, longitudinal study. Ann Surg Oncol. 2015; 22:3846–3852.
Article22. Rosenberg SM, Sepucha K, Ruddy KJ, Tamimi RM, Gelber S, Meyer ME, et al. Local therapy decision-making and contralateral prophylactic mastectomy in young women with early-stage breast cancer. Ann Surg Oncol. 2015; 22:3809–3815.
Article23. Ingham SL, Sperrin M, Baildam A, Ross GL, Clayton R, Lalloo F, et al. Risk-reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Res Treat. 2013; 142:611–618.
Article24. Heemskerk-Gerritsen BA, Rookus MA, Aalfs CM, Ausems MG, Collée JM, Jansen L, et al. Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer. 2015; 136:668–677.25. Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998-2011. JAMA. 2014; 312:902–914.
Article26. Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010; 10:CD002748.
Article27. Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg. 2014; 260:1000–1010.28. Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. 2014; 348:g226.
Article