Korean J Physiol Pharmacol.
2017 Jul;21(4):449-456. 10.4196/kjpp.2017.21.4.449.
Beauvericin, a cyclic peptide, inhibits inflammatory responses in macrophages by inhibiting the NF-κB pathway
- Affiliations
-
- 1Department of Genetic Engineering, Sungkyunkwan University, Suwon 16419, Korea. jaecho@skku.edu
- 2School of Systems Biomedical Science, Soongsil University, Seoul 06978, Korea. kimmy@ssu.ac.kr
- KMID: 2384459
- DOI: http://doi.org/10.4196/kjpp.2017.21.4.449
Abstract
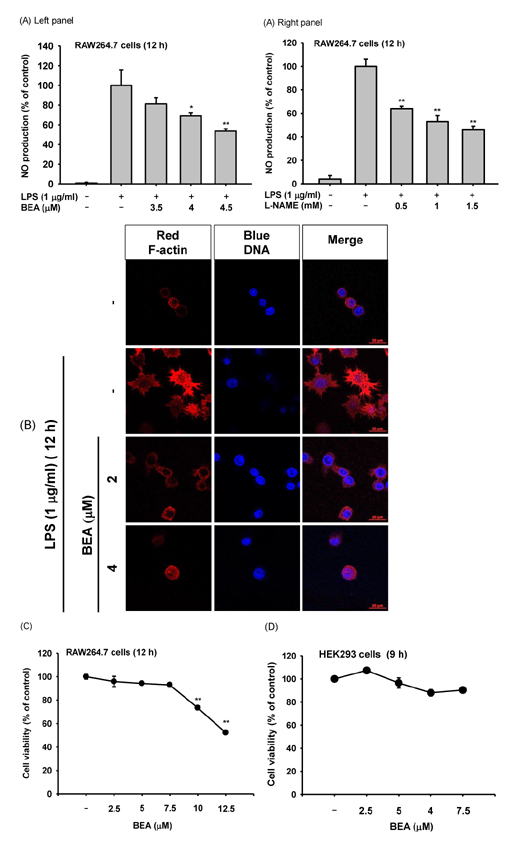
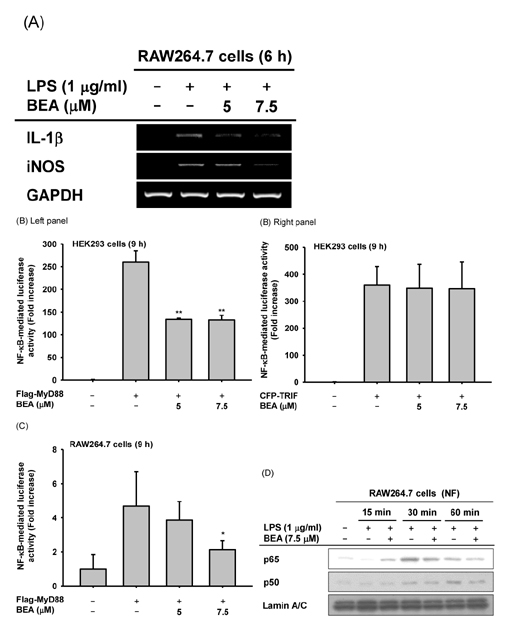
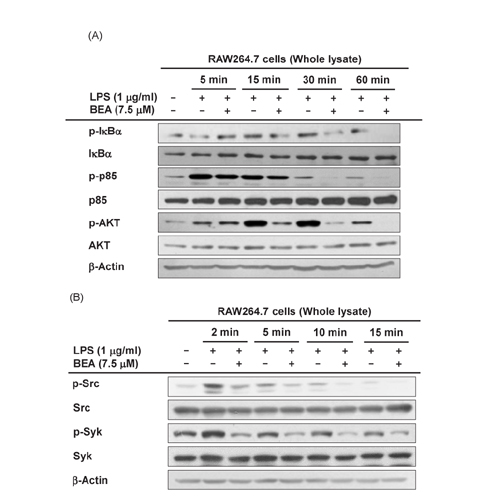
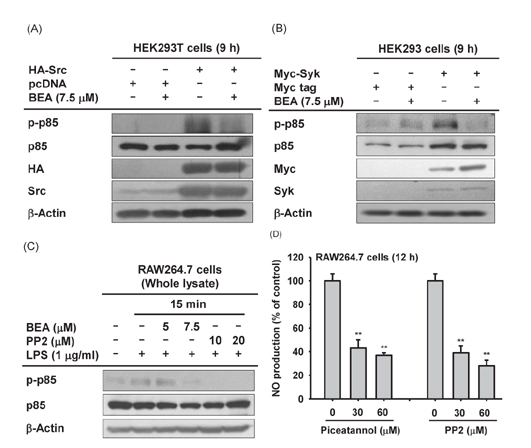
- Beauvericin (BEA), a cyclic hexadepsipeptide produced by the fungus Beauveria bassiana, is known to have anti-cancer, anti-inflammatory, and anti-microbial actions. However, how BEA suppresses macrophage-induced inflammatory responses has not been fully elucidated. In this study, we explored the anti-inflammatory properties of BEA and the underlying molecular mechanisms using lipopolysaccharide (LPS)-treated macrophage-like RAW264.7 cells. Levels of nitric oxide (NO), mRNA levels of transcription factors and the inflammatory genes inducible NO synthase (iNOS) and interleukin (IL)-1, and protein levels of activated intracellular signaling molecules were determined by Griess assay, semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR), luciferase reporter gene assay, and immunoblotting analysis. BEA dose-dependently blocked the production of NO in LPS-treated RAW264.7 cells without inducing cell cytotoxicity. BEA also prevented LPS-triggered morphological changes. This compound significantly inhibited nuclear translocation of the NF-κB subunits p65 and p50. Luciferase reporter gene assays demonstrated that BEA suppresses MyD88-dependent NF-κB activation. By analyzing upstream signaling events for NF-κB activation and overexpressing Src and Syk, these two enzymes were revealed to be targets of BEA. Together, these results suggest that BEA suppresses NF-κB-dependent inflammatory responses by suppressing both Src and Syk.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Formosanin C attenuates lipopolysaccharide-induced inflammation through nuclear factor-κB inhibition in macrophages
Limin Yin, Chaohong Shi, Zhongchen Zhang, Wensheng Wang, Ming Li
Korean J Physiol Pharmacol. 2021;25(5):395-401. doi: 10.4196/kjpp.2021.25.5.395.
Reference
-
1. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998; 282:2085–2088.2. Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004; 16:3–9.3. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140:805–820.4. Yi YS, Son YJ, Ryou C, Sung GH, Kim JH, Cho JY. Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014; DOI: 10.1155/2014/270302.5. Ohtsubo M, Okazaki H, Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989; 109:1389–1397.6. Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003; 278:24233–24241.7. Yang Y, Lee J, Rhee MH, Yu T, Baek KS, Sung NY, Kim Y, Yoon K, Kim JH, Kwak YS, Hong S, Kim JH, Cho JY. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015; 39:61–68.8. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–867.9. Jeon J, Kim Y, Kim H, Kang JS, Lee WJ. Anti-inflammatory effect of alloferon on ovalbumin-induced asthma. Immune Netw. 2015; 15:304–312.10. Morgan MJ, Kim YS. The serine threonine kinase RIP3: lost and found. BMB Rep. 2015; 48:303–312.11. Logrieco A, Moretti A, Castella G, Kostecki M, Golinski P, Ritieni A, Chelkowski J. Beauvericin production by Fusarium species. Appl Environ Microbiol. 1998; 64:3084–3088.12. Ovchinnikov YA, Ivanov VT, Mikhaleva II. The synthesis and some properties of beauvericin. Tetrahedron Lett. 1971; (2):159–162.13. Grove JF, Pople M. The insecticidal activity of beauvericin and the enniatin complex. Mycopathologia. 1980; 70:103–105.14. Fornelli F, Minervini F, Logrieco A. Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF-9). J Invertebr Pathol. 2004; 85:74–79.15. Castlebury L, Sutherland J, Tanner L, Henderson A, Cerniglia C. Use of a bioassay to evaluate the toxicity of beauvericin to bacteria. World J Microbiol Biotechnol. 1999; 15:119–121.16. Wang Q, Xu L. Beauvericin, a bioactive compound produced by fungi: a short review. Molecules. 2012; 17:2367–2377.17. Zhan J, Burns AM, Liu MX, Faeth SH, Gunatilaka AA. Search for cell motility and angiogenesis inhibitors with potential anticancer activity: beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. J Nat Prod. 2007; 70:227–232.18. Wu XF, Xu R, Ouyang ZJ, Qian C, Shen Y, Wu XD, Gu YH, Xu Q, Sun Y. Beauvericin ameliorates experimental colitis by inhibiting activated T cells via downregulation of the PI3K/Akt signaling pathway. PLoS One. 2013; 8:e83013.19. Wätjen W, Debbab A, Hohlfeld A, Chovolou Y, Proksch P. The mycotoxin beauvericin induces apoptotic cell death in H4IIE hepatoma cells accompanied by an inhibition of NF-κB-activity and modulation of MAP-kinases. Toxicol Lett. 2014; 231:9–16.20. Baek KS, Yi YS, Son YJ, Yoo S, Sung NY, Kim Y, Hong S, Aravinthan A, Kim JH, Cho JY. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016; 40:437–444.21. Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000; 398:399–407.22. Jung YY, Hong JT, Han SB, Park YH, Son DJ. Effect of Ixeris dentata Nakai extract on nitric oxide production and prostaglandin E2 generation in LPS-stimulated RAW264.7 Cells. Immune Netw. 2015; 15:325–330.23. Schrader M, Bahlmann K, Giese G, Hell SW. 4Pi-confocal imaging in fixed biological specimens. Biophys J. 1998; 75:1659–1668.24. Latt SA, Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem. 1976; 24:24–33.25. Jeon HJ, You SY, Park YS, Chang JW, Kim JS, Oh JS. TCTP regulates spindle microtubule dynamics by stabilizing polar microtubules during mouse oocyte meiosis. Biochim Biophys Acta. 2016; 1863:630–637.26. Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986; 94:57–63.27. Kim JH, Kim MY, Kim JH, Cho JY. Fisetin suppresses macrophage-mediated inflammatory responses by blockade of Src and Syk. Biomol Ther (Seoul). 2015; 23:414–420.28. Kim MS, Lee Y, Sung GH, Kim JH, Park JG, Kim HG, Baek KS, Cho JH, Han J, Lee KH, Hong S, Kim JH, Cho JY. Pro-apoptotic activity of 4-isopropyl-2-(1-phenylethyl) aniline isolated from Cordyceps bassiana. Biomol Ther (Seoul). 2015; 23:367–373.29. Bak MJ, Truong VL, Ko SY, Nguyen XN, Jun M, Hong SG, Lee JW, Jeong WS. Induction of Nrf2/ARE-mediated cytoprotective genes by red ginseng oil through ASK1-MKK4/7-JNK and p38 MAPK signaling pathways in HepG2 cells. J Ginseng Res. 2016; 40:423–430.30. Jung KK, Lee HS, Cho JY, Shin WC, Rhee MH, Kim TG, Kang JH, Kim SH, Hong S, Kang SY. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006; 79:2022–2031.31. Yu T, Ahn HM, Shen T, Yoon K, Jang HJ, Lee YJ, Yang HM, Kim JH, Kim C, Han MH, Cha SH, Kim TW, Kim SY, Lee J, Cho JY. Antiinflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011; 137:1197–1206.32. Byeon SE, Lee YG, Kim BH, Shen T, Lee SY, Park HJ, Park SC, Rhee MH, Cho JY. Surfactin blocks NO production in lipopolysaccharide-activated macrophages by inhibiting NF-kappaB activation. J Microbiol Biotechnol. 2008; 18:1984–1989.33. Cho JY, Choi GJ, Son SW, Jang KS, Lim HK, Lee SO, Sung ND, Cho KY, Kim JC. Isolation and antifungal activity of lignans from Myristica fragrans against various plant pathogenic fungi. Pest Manag Sci. 2007; 63:935–940.34. Sung NY, Kim MY, Cho JY. Scutellarein reduces inflammatory responses by inhibiting Src kinase activity. Korean J Physiol Pharmacol. 2015; 19:441–449.35. Yang WS, Ratan ZA, Kim G, Lee Y, Kim MY, Kim JH, Cho JY. 4-Isopropyl-2,6-bis(1-phenylethyl)aniline 1, an analogue of KTH-13 isolated from Cordyceps bassiana, inhibits the NF-κB-mediated inflammatory response. Mediators Inflamm. 2015; DOI: 10.1155/2015/143025.36. Baek KS, Hong YD, Kim Y, Sung NY, Yang S, Lee KM, Park JY, Park JS, Rho HS, Shin SS, Cho JY. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015; 39:155–161.37. Kim S, Oh MH, Kim BS, Kim WI, Cho HS, Park BY, Park C, Shin GW, Kwon J. Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J Ginseng Res. 2015; 39:365–370.38. Hossen MJ, Jeon SH, Kim SC, Kim JH, Jeong D, Sung NY, Yang S, Baek KS, Kim JH, Yoon DH, Song WO, Yoon KD, Cho SH, Lee S, Kim JH, Cho JY. In vitro and in vivo anti-inflammatory activity of Phyllanthus acidus methanolic extract. J Ethnopharmacol. 2015; 168:217–228.39. Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013; 12:86.40. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009; 1:a001651.41. Caamaño J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002; 15:414–429.42. Lee YG, Chain BM, Cho JY. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of kappaB alpha via activation of the phosphoinositide-3-kinase and Akt pathways. Int J Biochem Cell Biol. 2009; 41:811–821.43. Lee JY, Lee YG, Lee J, Yang KJ, Kim AR, Kim JY, Won MH, Park J, Yoo BC, Kim S, Cho WJ, Cho JY. Akt Cys-310-targeted inhibition by hydroxylated benzene derivatives is tightly linked to their immunosuppressive effects. J Biol Chem. 2010; 285:9932–9948.44. Murphy M, Xiong Y, Pattabiraman G, Qiu F, Medvedev AE. Pellino-1 positively regulates toll-like receptor (TLR) 2 and TLR4 signaling and is suppressed upon induction of endotoxin tolerance. J Biol Chem. 2015; 290:19218–19232.45. Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007; 85:249–256.46. Ko R, Lee SY. Glycogen synthase kinase 3β in Toll-like receptor signaling. BMB Rep. 2016; 49:305–310.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of CpG-ODN-Induced TLR9 Signaling Inhibited by Interleukin-37 in U937 Human Macrophages
- Rhodanthpyrone A and B play an anti-inflammatory role by suppressing the nuclear factor-κB pathway in macrophages
- Triptolide-induced Transrepression of IL-8 NF-kappaB in Lung Epithelial Cells
- Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- Aloe-emodin inhibits Pam₃CSK₄-induced MAPK and NF-κB signaling through TLR2 in macrophages