Korean J Physiol Pharmacol.
2017 Jul;21(4):429-437. 10.4196/kjpp.2017.21.4.429.
Relaxant and anti-inflammatory effect of two thalidomide analogs as PDE-4 inhibitors in pregnant rat uterus
- Affiliations
-
- 1Center for Research on Reproductive Biology, Academic Area of Medicine, Institute of Health Sciences, Autonomous University of Hidalgo's State, Pachuca, Hidalgo 42090, Mexico. tomedyfm@hotmail.com, efernan@uaeh.edu.mx
- KMID: 2384457
- DOI: http://doi.org/10.4196/kjpp.2017.21.4.429
Abstract
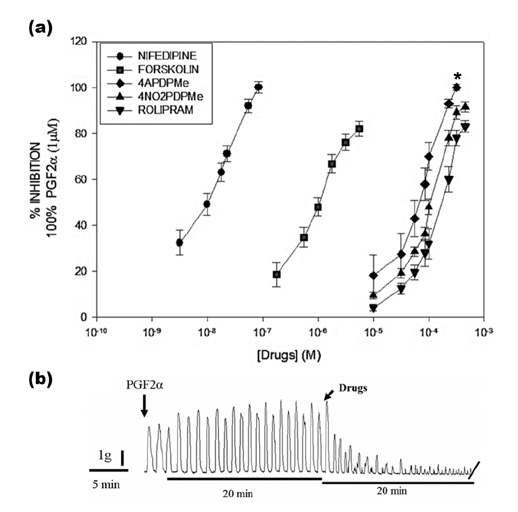
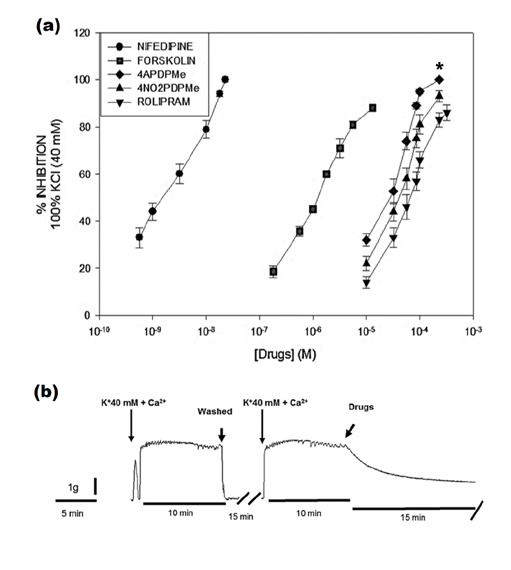
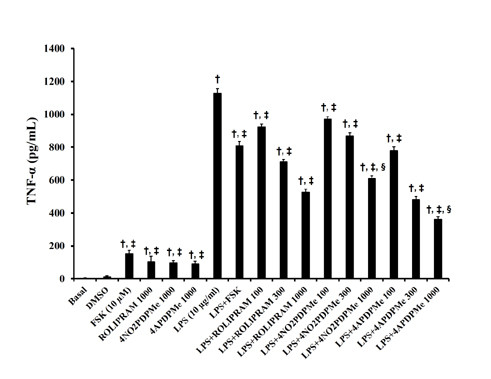
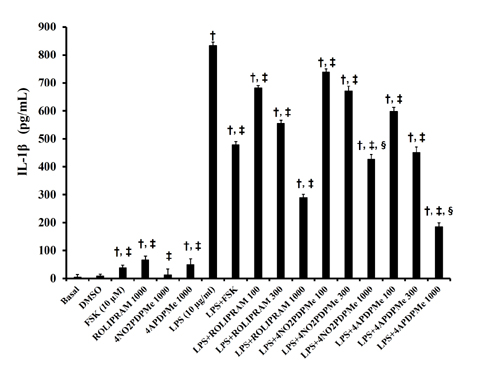
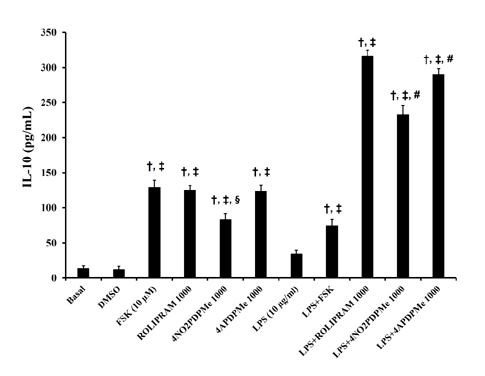
- The aim of this study was to evaluate the relaxant and anti-inflammatory effects of two thalidomide analogs as phosphodiesterase-4 (PDE-4) inhibitors in pregnant rat uterus. Uteri from Wistar female rats were isolated at 19 day of pregnancy. Uterine samples were used in functional studies to evaluate the inhibitory effects of the thalidomide analogs, methyl 3-(4-nitrophthalimido)-3-(3,4-dimethoxyphenyl)-propanoate (4NO2PDPMe) and methyl 3-(4-aminophthalimido)-3-(3,4-dimethoxyphenyl)-propanoate (4APDPMe), on prostaglandin-F2α (PGF2α)-induced phasic, Kâº-induced tonic, and Ca²âº-induced contractions. Accumulation of cAMP was quantified in uterine homogenates by ELISA. Anti-inflammatory effect was assessed by using ELISA for determination of the pro-inflammatory cytokines tumor necrosis factor-α (TNFα) and interleukin (IL)-1β, and anti-inflammatory IL-10, from uterine explants stimulated with lipopolysaccharide (LPS). Nifedipine, forskolin and rolipram were used as positive controls where required. Both thalidomide analogs induced a significant inhibition of the uterine contractions induced by the pharmaco- and electro-mechanic stimuli. Nifedipine and forskolin were more potent than the analogs to inhibit the uterine contractility, but these were more potent than rolipram, and 4APDPMe was equieffective to nifedipine. Thalidomide analogs increased uterine cAMP-levels in a concentration-dependent manner. The LPS-induced TNFα and IL-1β uterine secretion was diminished in a concentration-dependent fashion by both analogs, whereas IL-10 secretion was increased significantly. The thalidomide analogs induced utero-relaxant and anti-inflammatory effects, which were associated with the increased cAMP levels as PDE-4 inhibitors in the pregnant rat uterus. Such properties place these thalidomide analogs as potentially safe and effective tocolytic agents in a field that urgently needs improved pharmacological treatments, as in cases of preterm labor.
MeSH Terms
-
Animals
Colforsin
Cyclic Nucleotide Phosphodiesterases, Type 4
Cytokines
Enzyme-Linked Immunosorbent Assay
Female
Humans
Interleukin-10
Interleukins
Necrosis
Nifedipine
Obstetric Labor, Premature
Phosphodiesterase 4 Inhibitors*
Pregnancy
Rats*
Rolipram
Thalidomide*
Tocolytic Agents
Uterine Contraction
Uterus*
Colforsin
Cyclic Nucleotide Phosphodiesterases, Type 4
Cytokines
Interleukin-10
Interleukins
Nifedipine
Phosphodiesterase 4 Inhibitors
Rolipram
Thalidomide
Tocolytic Agents
Figure
Cited by 1 articles
-
Anti-inflammatory and utero-relaxant effect of α-bisabolol on the pregnant human uterus
Victor Manuel Muñoz-Pérez, Mario I. Ortiz, Héctor A. Ponce-Monter, Vicente Monter-Pérez, Guillermo Barragán-Ramírez
Korean J Physiol Pharmacol. 2018;22(4):391-398. doi: 10.4196/kjpp.2018.22.4.391.
Reference
-
1. Méhats C, Oger S, Leroy MJ. Cyclic nucleotide phosphodiesterase-4 inhibitors: a promising therapeutic approach to premature birth? Eur J Obstet Gynecol Reprod Biol. 2004; 117:Suppl 1. S15–S17.2. Sehringer B, Schäfer WR, Wetzka B, Deppert WR, Brunner-Spahr R, Benedek E, Zahradnik HP. Formation of proinflammatory cytokines in human term myometrium is stimulated by lipopolysaccharide but not by corticotropin-releasing hormone. J Clin Endocrinol Metab. 2000; 85:4859–4865.3. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000; 342:1500–1507.4. Pollard JK, Mitchell MD. Intrauterine infection and the effects of inflammatory mediators on prostaglandin production by myometrial cells from pregnant women. Am J Obstet Gynecol. 1996; 174:682–686.5. Sumi G, Yasuda K, Kanamori C, Kajimoto M, Nishigaki A, Tsuzuki T, Cho H, Okada H, Kanzaki H. Two-step inhibitory effect of kanzo on oxytocin-induced and prostaglandin F2α-induced uterine myometrial contractions. J Nat Med. 2014; 68:550–560.6. Fernández-Martínez E, Ponce-Monter H, Soria-Jasso LE, Ortiz MI, Arias-Montaño JA, Barragán-Ramírez G, Mayén-García C. Inhibition of uterine contractility by thalidomide analogs via phosphodiesterase-4 inhibition and calcium entry blockade. Molecules. 2016; 21:E1332.7. Méhats C, Schmitz T, Oger S, Hervé R, Cabrol D, Leroy MJ. PDE4 as a target in preterm labour. BMC Pregnancy Childbirth. 2007; 7:Suppl 1. S12.8. Bäumer W, Hoppmann J, Rundfeldt C, Kietzmann M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets. 2007; 6:17–26.9. Krymskaya VP, Panettieri RA Jr. Phosphodiesterases regulate airway smooth muscle function in health and disease. Curr Top Dev Biol. 2007; 79:61–74.10. Leroy MJ, Méhats C, Duc-Goiran P, Tanguy G, Robert B, Dallot E, Mignot TM, Grangé G, Ferré F. Effect of pregnancy on PDE4 cAMP-specific phosphodiesterase messenger ribonucleic acid expression in human myometrium. Cell Signal. 1999; 11:31–37.11. Boswell-Smith V, Spina D. PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int J Chron Obstruct Pulmon Dis. 2007; 2:121–129.12. Schmitz T, Souil E, Hervé R, Nicco C, Batteux F, Germain G, Cabrol D, Evain-Brion D, Leroy MJ, Méhats C. PDE4 inhibition prevents preterm delivery induced by an intrauterine inflammation. J Immunol. 2007; 178:1115–1121.13. Oger S, Méhats C, Barnette MS, Ferré F, Cabrol D, Leroy MJ. Anti-inflammatory and utero-relaxant effects in human myometrium of new generation phosphodiesterase 4 inhibitors. Biol Reprod. 2004; 70:458–464.14. Oger S, Méhats C, Dallot E, Ferré F, Leroy MJ. Interleukin-1beta induces phosphodiesterase 4B2 expression in human myometrial cells through a prostaglandin E2- and cyclic adenosine 3',5'-monophosphate-dependent pathway. J Clin Endocrinol Metab. 2002; 87:5524–5531.15. Hubinont C, Debieve F. Prevention of preterm labour: 2011 update on tocolysis. J Pregnancy. 2011; 2011:941057.16. de Heus R, Mol BW, Erwich JJ, van Geijn HP, Gyselaers WJ, Hanssens M, Härmark L, van Holsbeke CD, Duvekot JJ, Schobben FF, Wolf H, Visser GH. Adverse drug reactions to tocolytic treatment for preterm labour: prospective cohort study. BMJ. 2009; 338:b744.17. Verli J, Klukovits A, Kormányos Z, Hajagos-Tóth J, Ducza E, Seres AB, Falkay G, Gáspár R. Uterus-relaxing effect of β2-agonists in combination with phosphodiesterase inhibitors: studies on pregnant rat in vivo and on pregnant human myometrium in vitro. J Obstet Gynaecol Res. 2013; 39:31–39.18. Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999; 163:380–386.19. Man HW, Schafer P, Wong LM, Patterson RT, Corral LG, Raymon H, Blease K, Leisten J, Shirley MA, Tang Y, Babusis DM, Chen R, Stirling D, Muller GW. Discovery of (S)-N-[2-[1-(3-ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl] acetamide (apremilast), a potent and orally active phosphodiesterase 4 and tumor necrosis factor-alpha inhibitor. J Med Chem. 2009; 52:1522–1524.20. Marriott JB, Westby M, Cookson S, Guckian M, Goodbourn S, Muller G, Shire MG, Stirling D, Dalgleish AG. CC-3052: a water-soluble analog of thalidomide and potent inhibitor of activation-induced TNF-alpha production. J Immunol. 1998; 161:4236–4243.21. Muller GW, Corral LG, Shire MG, Wang H, Moreira A, Kaplan G, Stirling DI. Structural modifications of thalidomide produce analogs with enhanced tumor necrosis factor inhibitory activity. J Med Chem. 1996; 39:3238–3240.22. Muller GW, Shire MG, Wong LM, Corral LG, Patterson RT, Chen Y, Stirling DI. Thalidomide analogs and PDE4 inhibition. Bioorg Med Chem Lett. 1998; 8:2669–2674.23. Gáspár R, Gál A, Gálik M, Ducza E, Minorics R, Kolarovszki-Sipiczki Z, Klukovits A, Falkay G. Different roles of alpha2-adrenoceptor subtypes in non-pregnant and late-pregnant uterine contractility in vitro in the rat. Neurochem Int. 2007; 51:311–318.24. Fernández-Martínez E, Pérez-Hernández N, Muriel P, Pérez Alvarez V, Shibayama M, Tsutsumi V. The thalidomide analog 3-phthalimido-3-(3,4-dimethoxyphenyl)-propanoic acid improves the biliary cirrhosis in the rat. Exp Toxicol Pathol. 2009; 61:471–479.25. Klukovits A, Ducza E, Földesi I, Falkay G. Beta 2-agonist treatment enhances uterine oxytocin receptor mRNA expression in pregnant rats. Mol Reprod Dev. 2004; 69:60–65.26. Sato TA, Keelan JA, Mitchell MD. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J Immunol. 2003; 170:158–166.27. Schafer PH, Parton A, Capone L, Cedzik D, Brady H, Evans JF, Man HW, Muller GW, Stirling DI, Chopra R. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014; 26:2016–2029.28. Inoue Y, Shimamura K, Sperelakis N. Forskolin inhibition of K+ current in pregnant rat uterine smooth muscle cells. Eur J Pharmacol. 1993; 240:169–176.29. Katz AM. Pharmacology and mechanisms of action of calcium-channel blockers. J Clin Hypertens. 1986; 2:3 Suppl. 28S–37S.30. Méhats C, Tanguy G, Dallot E, Robert B, Rebourcet R, Ferre F, Leroy MJ. Selective up-regulation of phosphodiesterase-4 cyclic adenosine 3',5'-monophosphate (cAMP)-specific phosphodiesterase variants by elevated cAMP content in human myometrial cells in culture. Endocrinology. 1999; 140:3228–3237.31. Lubomirov LT, Reimann K, Metzler D, Hasse V, Stehle R, Ito M, Hartshorne DJ, Gagov H, Pfitzer G, Schubert R. Urocortin-induced decrease in Ca2+ sensitivity of contraction in mouse tail arteries is attributable to cAMP-dependent dephosphorylation of MYPT1 and activation of myosin light chain phosphatase. Circ Res. 2006; 98:1159–1167.32. Aguilar HN, Tracey CN, Zielnik B, Mitchell BF. Rho-kinase mediates diphosphorylation of myosin regulatory light chain in cultured uterine, but not vascular smooth muscle cells. J Cell Mol Med. 2012; 16:2978–2989.33. Hudson CA, Heesom KJ, López Bernal A. Phasic contractions of isolated human myometrium are associated with Rho-kinase (ROCK)-dependent phosphorylation of myosin phosphatase-targeting subunit (MYPT1). Mol Hum Reprod. 2012; 18:265–279.34. Shmygol A, Blanks AM, Bru-Mercier G, Gullam JE, Thornton S. Control of uterine Ca2+ by membrane voltage: toward understanding the excitation-contraction coupling in human myometrium. Ann N Y Acad Sci. 2007; 1101:97–109.35. Azam MA, Tripuraneni NS. Selective phosphodiesterase 4B inhibitors: a review. Sci Pharm. 2014; 82:453–481.36. Revuelta MP, Cantabrana B, Hidalgo A. Pharmacological evidence for the contribution of polyamines as mediators of the transcriptional component involved in smooth muscle relaxation elicited by forskolin. Life Sci. 1997; 61:2443–2454.37. Sim MK. Effect of IUD on uterine cyclic AMP and the activities of adenyl cyclase and phosphodiesterase during the oestrous cycle and early pregnancy in rats. J Reprod Fertil. 1974; 39:399–402.38. Bizargity P, Del Rio R, Phillippe M, Teuscher C, Bonney EA. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol Reprod. 2009; 80:874–881.39. Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014; 11:571–581.40. Gantner F, Kupferschmidt R, Schudt C, Wendel A, Hatzelmann A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumour necrosis factor-alpha release by PDE inhibitors. Br J Pharmacol. 1997; 121:221–231.41. Yoshida N, Shimizu Y, Kitaichi K, Hiramatsu K, Takeuchi M, Ito Y, Kume H, Yamaki K, Suzuki R, Shibata E, Hasegawa T, Takagi K. Differential effect of phosphodiesterase inhibitors on IL-13 release from peripheral blood mononuclear cells. Clin Exp Immunol. 2001; 126:384–389.42. Gerlo S, Kooijman R, Beck IM, Kolmus K, Spooren A, Haegeman G. Cyclic AMP: a selective modulator of NF-κB action. Cell Mol Life Sci. 2011; 68:3823–3841.43. Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, Simon MI, Fraser ID. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009; 2:ra28.44. Hervé R, Schmitz T, Evain-Brion D, Cabrol D, Leroy MJ, Méhats C. The PDE4 inhibitor rolipram prevents NF-kappaB binding activity and proinflammatory cytokine release in human chorionic cells. J Immunol. 2008; 181:2196–2202.45. Jin SL, Ding SL, Lin SC. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang Gung Med J. 2012; 35:197–210.46. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012; 32:23–63.47. Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010; 10:170–181.48. Fernández-Martínez E, Morales-Ríos MS, Pérez-Álvarez V, Muriel P. Effects of thalidomide and 3-phthalimido-3-(3,4-dimethoxyphenyl)-propanamide on bile duct obstruction-induced cirrhosis in the rat. Drug Development Research. 2001; 54:209–218.49. Klukovits A, Verli J, Falkay G, Gáspár R. Improving the relaxing effect of terbutaline with phosphodiesterase inhibitors: studies on pregnant rat uteri in vitro. Life Sci. 2010; 87:733–737.50. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015; 75:1393–1403.51. Guariento S, Bruno O, Fossa P, Cichero E. New insights into PDE4B inhibitor selectivity: CoMFA analyses and molecular docking studies. Mol Divers. 2016; 20:77–92.52. Del Rosso JQ, Kircik L. Oral apremilast for the treatment of plaque psoriasis. J Clin Aesthet Dermatol. 2016; 9:43–48.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thalidomide and a Dipeptidyl Peptidase 4 Inhibitor in a Rat Model of Experimental Autoimmune Myocarditis
- Anti-inflammatory and utero-relaxant effect of α-bisabolol on the pregnant human uterus
- Synergistic anti-allodynic effect between intraperitoneal thalidomide and morphine on rat spinal nerve ligation-induced neuropathic pain
- Effect of Thalidomide on the Angiogenesis and In vivo Growth of Hepatoma Cells in the Mouse
- The Effect of PDE 4 Inhibitor on Urinary Bladder Function in Normal Rats