Korean J Physiol Pharmacol.
2017 Jul;21(4):361-370. 10.4196/kjpp.2017.21.4.361.
Effects of tianeptine on symptoms of fibromyalgia via BDNF signaling in a fibromyalgia animal model
- Affiliations
-
- 1Department of Clinical Pharmacology, College of Medicine, Soonchunhyang University, Cheonan 31151, Korea. hak3962@sch.ac.kr
- 2Department of Integrative Plant Science, Chung-Ang University, Anseong 17546, Korea.
- 3Department of Convergence Medical Science, Brain Korea 21 Plus Program, and Institute of Korean Medicine, College of Oriental Medicine, Kyung Hee University, Seoul 02453, Korea.
- 4Development of Ginseng and Medical Plants Research Institute, Rural Administration, Eumseong 27709, Korea.
- 5Soonchunhyang Medical Research Institute, College of Medicine, Soonchunhyang University, Cheonan 31151, Korea.
- KMID: 2384449
- DOI: http://doi.org/10.4196/kjpp.2017.21.4.361
Abstract
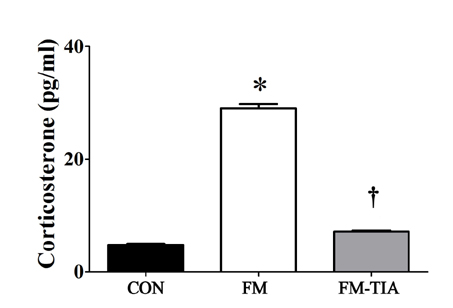
- Previous reports have suggested that physical and psychological stresses may trigger fibromyalgia (FM). Stress is an important risk factor in the development of depression and memory impairments. Antidepressants have been used to prevent stress-induced abnormal pain sensation. Among various antidepressants, tianeptine has been reported to be able to prevent neurodegeneration due to chronic stress and reverse decreases in hippocampal volume. To assess the possible effect of tianeptine on FM symptoms, we constructed a FM animal model induced by restraint stress with intermittent cold stress. All mice underwent nociceptive assays using electronic von Frey anesthesiometer and Hargreaves equipment. To assess the relationship between tianeptine and expression levels of brain-derived neurotrophic factor (BDNF), cAMP response element-binding protein (CREB), and phosphorylated cAMP response element-binding protein (p-CREB), western blotting and immunohistochemistry analyses were performed. In behavioral analysis, nociception tests showed that pain threshold was significantly decreased in the FM group compared to that in the control group. Western blot and immunohistochemical analyses of medial prefrontal cortex (mPFC) and hippocampus showed downregulation of BDNF and p-CREB proteins in the FM group compared to the control group. However, tianeptine recovered these changes in behavioral tests and protein level. Therefore, this FM animal model might be useful for investigating mechanisms linking BDNF-CREB pathway and pain. Our results suggest that tianeptine might potentially have therapeutic efficacy for FM.
Keyword
MeSH Terms
-
Animals*
Antidepressive Agents
Behavior Rating Scale
Blotting, Western
Brain-Derived Neurotrophic Factor*
Cyclic AMP Response Element-Binding Protein
Depression
Down-Regulation
Fibromyalgia*
Hippocampus
Immunohistochemistry
Memory
Mice
Models, Animal*
Pain Measurement
Pain Threshold
Prefrontal Cortex
Risk Factors
Sensation
Stress, Psychological
Antidepressive Agents
Brain-Derived Neurotrophic Factor
Cyclic AMP Response Element-Binding Protein
Figure
Reference
-
1. Clauw DJ. Fibromyalgia: more than just a musculoskeletal disease. Am Fam Physician. 1995; 52:843–851. 853–854.2. Rohrbeck J, Jordan K, Croft P. The frequency and characteristics of chronic widespread pain in general practice: a case-control study. Br J Gen Pract. 2007; 57:109–115.3. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992; 267:1244–1252.4. Bardin L, Malfetes N, Newman-Tancredi A, Depoortère R. Chronic restraint stress induces mechanical and cold allodynia, and enhances inflammatory pain in rat: Relevance to human stress-associated painful pathologies. Behav Brain Res. 2009; 205:360–366.5. Nishiyori M, Ueda H. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol Pain. 2008; 4:52.6. Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995; 69:89–98.7. Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009; 12:167–177.8. Goffer Y, Xu D, Eberle SE, D'amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci. 2013; 33:19034–19044.9. Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012; 32:5747–5756.10. Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010; 15:404–414.11. De Ryck LM, Raus JC. Superfusion of normal and neoplastic mouse mammary tissue with estrogens. Endocrinology. 1983; 113:399–408.12. Arnold LM, Bradley LA, Clauw DJ, Glass JM, Goldenberg DL. Multidisciplinary care and stepwise treatment for fibromyalgia. J Clin Psychiatry. 2008; 69:e35.13. Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE Jr, Welge JA, Bishop F, Stanford KE, Hess EV, Hudson JI. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007; 56:1336–1344.14. Arnold LM, Russell IJ, Diri EW, Duan WR, Young JP Jr, Sharma U, Martin SA, Barrett JA, Haig G. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain. 2008; 9:792–805.15. M'Dahoma S, Barthélemy S, Tromilin C, Jeanson T, Viguier F, Michot B, Pezet S, Hamon M, Bourgoin S. Respective pharmacological features of neuropathic-like pain evoked by intrathecal BDNF versus sciatic nerve ligation in rats. Eur Neuropsychopharmacol. 2015; 25:2118–2130.16. Schmidt-Kastner R, Wetmore C, Olson L. Comparative study of brain-derived neurotrophic factor messenger RNA and protein at the cellular level suggests multiple roles in hippocampus, striatum and cortex. Neuroscience. 1996; 74:161–183.17. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006; 59:1116–1127.18. Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003; 54:70–75.19. McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005; 54:5 Suppl 1. 20–23.20. Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, Mannari C, Martini C, Da Pozzo E, Schiavi E, Mariotti A, Roncaglia I, Palla A, Consoli G, Giovannini L, Massimetti G, Dell'Osso L. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2008; 105:279–283.21. Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008; 64:527–532.22. Miletic G, Miletic V. Increases in the concentration of brain derived neurotrophic factor in the lumbar spinal dorsal horn are associated with pain behavior following chronic constriction injury in rats. Neurosci Lett. 2002; 319:137–140.23. Guo JQ, Deng HH, Bo X, Yang XS. Involvement of BDNF/TrkB and ERK/CREB axes in nitroglycerin-induced rat migraine and effects of estrogen on these signals in the migraine. Biol Open. 2017; 6:8–16.24. Bhatt DK, Ramachandran R, Christensen SL, Gupta S, Jansen-Olesen I, Olesen J. CGRP infusion in unanesthetized rats increases expression of c-Fos in the nucleus tractus solitarius and caudal ventrolateral medulla, but not in the trigeminal nucleus caudalis. Cephalalgia. 2015; 35:220–233.25. Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006; 99:1338–1350.26. Yajima Y, Narita M, Narita M, Matsumoto N, Suzuki T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res. 2002; 958:338–346.27. Clauw DJ. Fibromyalgia: an overview. Am J Med. 2009; 122:12 Suppl. 3S–13S.28. Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav. 1997; 56:131–137.29. Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002; 22:3251–3261.30. Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010; 35:2378–2391.31. Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995; 15:7539–7547.32. Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996; 16:2365–2372.33. McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, Fuchs E. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010; 15:237–249.34. Watson PN, Merskey H. Antidepressant analgesics in pain management. Encyclopedia of Pain. 2007. p. 100–104.35. Uzbay IT, Cinar MG, Aytemir M, Tuglular I. Analgesic effect of tianeptine in mice. Life Sci. 1999; 64:1313–1319.36. Chu CC, Wang JJ, Chen KT, Shieh JP, Wang LK, Shui HA, Ho ST. Neurotrophic effects of tianeptine on hippocampal neurons: a proteomic approach. J Proteome Res. 2010; 9:936–944.37. Kim WM, Lee SH, Jeong HJ, Lee HG, Choi JI, Yoon MH. The analgesic activity of intrathecal tianeptine, an atypical antidepressant, in a rat model of inflammatory pain. Anesth Analg. 2012; 114:683–689.38. Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001; 98:12796–12801.39. Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004; 101:3973–3978.40. Uzbay TI. Tianeptine: potential influences on neuroplasticity and novel pharmacological effects. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32:915–924.41. Defrance R, Marey C, Kamoun A. Antidepressant and anxiolytic activities of tianeptine: an overview of clinical trials. Clin Neuropharmacol. 1988; 11:Suppl 2. S74–S82.42. Guelfi JD, Pichot P, Dreyfus JF. Efficacy of tianeptine in anxious-depressed patients: results of a controlled multicenter trial versus amitriptyline. Neuropsychobiology. 1989; 22:41–48.43. Wilde MI, Benfield P. Tianeptine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depression and coexisting anxiety and depression. Drugs. 1995; 49:411–439.44. Ejchel-Cohen TF, Wood GE, Wang JF, Barlow K, Nobrega JN, S McEwen B, Trevor Young L. Chronic restraint stress decreases the expression of glutathione S-transferase pi2 in the mouse hippocampus. Brain Res. 2006; 1090:156–162.45. Magariños AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, McEwen BS. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011; 21:253–264.46. Nishiyori M, Uchida H, Nagai J, Araki K, Mukae T, Kishioka S, Ueda H. Permanent relief from intermittent cold stress-induced fibromyalgia-like abnormal pain by repeated intrathecal administration of antidepressants. Mol Pain. 2011; 7:69.47. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985; 85:367–370.48. Keyhanfar F, Shamsi Meymandi M, Sepehri G, Rastegaryanzadeh R, Heravi G. Evaluation of antinociceptive effect of pregabalin in mice and its combination with tramadol using tail flick test. Iran J Pharm Res. 2013; 12:483–493.49. Meymandi MS, Sepehri G, Mobasher M. Gabapentin enhances the analgesic response to morphine in acute model of pain in male rats. Pharmacol Biochem Behav. 2006; 85:185–189.50. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988; 32:77–88.51. Reddyjarugu B, Pavek T, Southard T, Barry J, Singh B. Analgesic efficacy of firocoxib, a selective inhibitor of cyclooxygenase 2, in a mouse model of incisional pain. J Am Assoc Lab Anim Sci. 2015; 54:405–410.52. Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004; 10:712–718.53. Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004; 309:380–387.54. Morley-Fletcher S, Darnaudery M, Koehl M, Casolini P, Van Reeth O, Maccari S. Prenatal stress in rats predicts immobility behavior in the forced swim test. Effects of a chronic treatment with tianeptine. Brain Res. 2003; 989:246–251.55. Joo J, Lee S, Nah SS, Kim YO, Kim DS, Shim SH, Hwangbo Y, Kim HK, Kwon JT, Kim JW, Song HY, Kim HJ. Lasp1 is down-regulated in NMDA receptor antagonist-treated mice and implicated in human schizophrenia susceptibility. J Psychiatr Res. 2013; 47:105–112.56. Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008; 85:297–317.57. Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006; 11:2179–2192.58. Nowacka MM, Paul-Samojedny M, Bielecka AM, Plewka D, Czekaj P, Obuchowicz E. LPS reduces BDNF and VEGF expression in the structures of the HPA axis of chronic social stressed female rats. Neuropeptides. 2015; 54:17–27.59. Duman RS, Vaidya VA. Molecular and cellular actions of chronic electroconvulsive seizures. J ECT. 1998; 14:181–193.60. Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011; 35:722–729.61. Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl). 2005; 179:769–780.62. Mizuki I, Sato A, Matsuo A, Suyama Y, Suzuki J, Makita A. Clonal structure, seed set, and self-pollination rate in mass-flowering bamboo species during off-year flowering events. PLoS One. 2014; 9:e105051.63. Duric V, McCarson KE. Effects of analgesic or antidepressant drugs on pain- or stress-evoked hippocampal and spinal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression in the rat. J Pharmacol Exp Ther. 2006; 319:1235–1243.64. Kremer M, Yalcin I, Nexon L, Wurtz X, Ceredig RA, Daniel D, Hawkes RA, Salvat E, Barrot M. The antiallodynic action of pregabalin in neuropathic pain is independent from the opioid system. Mol Pain. 2016; 12:1744806916633477.65. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007; (4):CD005454.66. Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005; 96:399–409.67. Sindrup SH, Gram LF, Brøsen K, Eshøj O, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990; 42:135–144.68. Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992; 326:1250–1256.69. Sindrup SH, Bjerre U, Dejgaard A, Brøsen K, Aaes-Jørgensen T, Gram LF. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992; 52:547–552.