Korean J Gastroenterol.
2016 Dec;68(6):317-320. 10.4166/kjg.2016.68.6.317.
A Case of Tenofovir-associated Fanconi Syndrome in Patient with Chronic Hepatitis B
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. 93cool@hanmail.net
- KMID: 2383462
- DOI: http://doi.org/10.4166/kjg.2016.68.6.317
Abstract
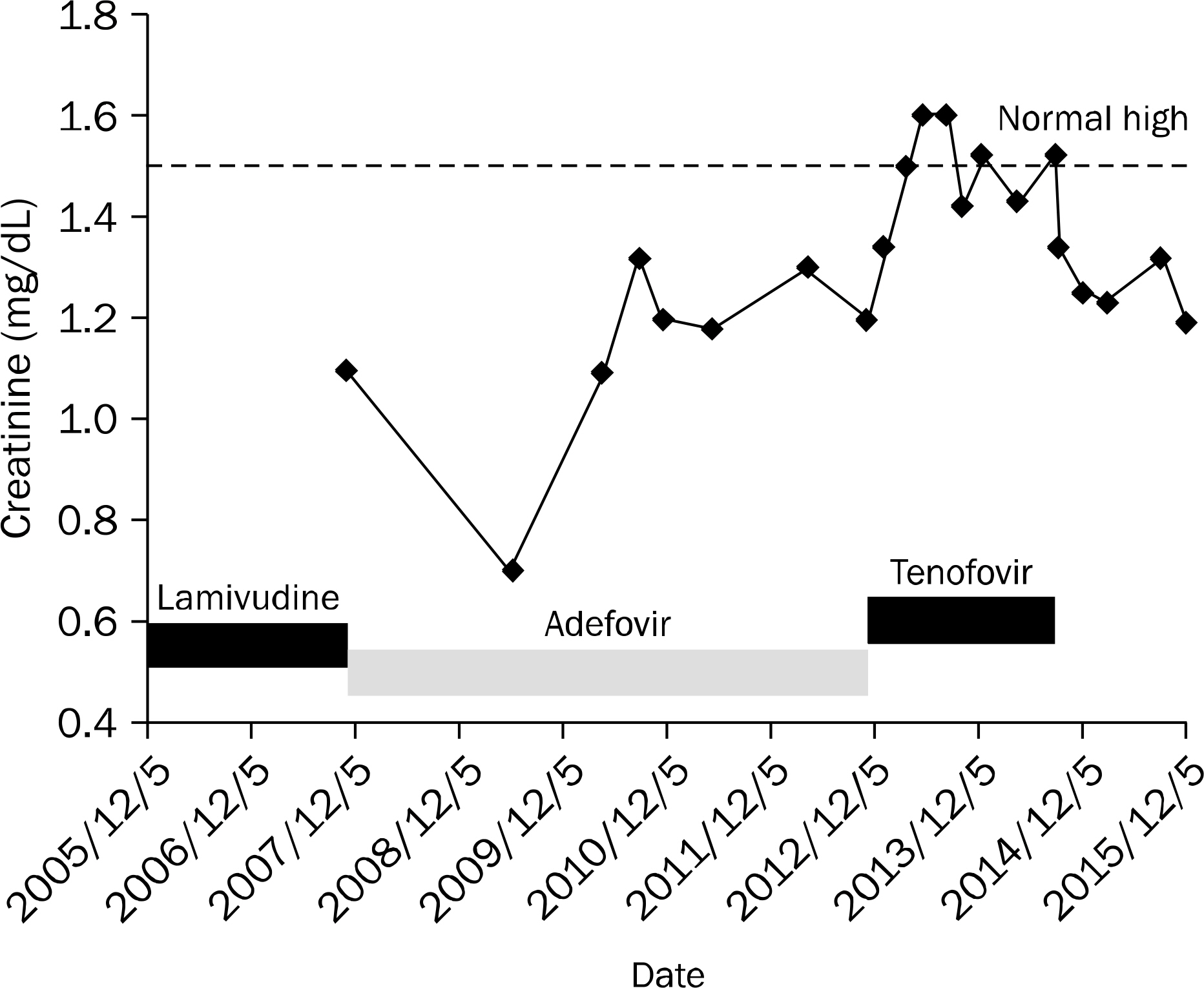
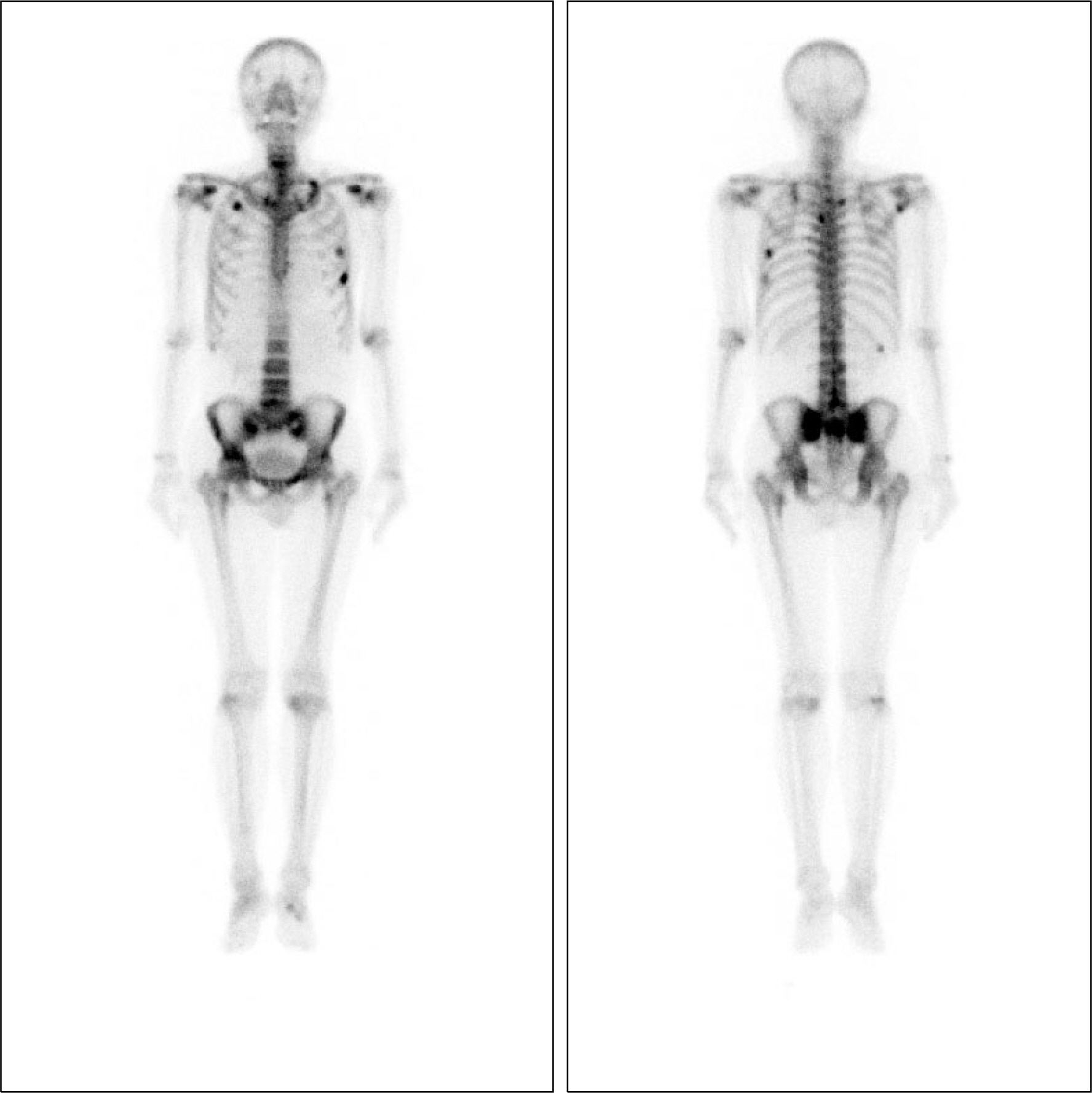
- Tenofovir disoproxil fumarate (TDF) is one of the most widely used treatment options for human immunodeficiency virus (HIV) and HBV infections. Despite its efficacy and safety, some cases of nephrotoxicity have been reported in the treatment of HIV patients. Even more recently, very few cases of Fanconi syndrome associated with tenofovir therapy in HBV monoinfection have been reported. Herein, we report a case of a 47-year-old male with an HBV monoinfection, who developed Fanconi syndrome and a secondary osteomalacia with multiple bone pain. After TDF withdrawal and supplementation of calcitriol, his renal function was reverted. Although the overall risk of TDF-associated nephrotoxicity is very low, both glomerular and tubular function should be monitored in patients undergoing TDF treatment.
MeSH Terms
Figure
Reference
-
References
1. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012; 57:167–185.2. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012; 35:1317–1325.
Article3. Jafari A, Khalili H, Dashti-Khavidaki S. Tenofovir-induced nephrotoxicity: incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur J Clin Pharmacol. 2014; 70:1029–1040.
Article4. Quinn KJ, Emerson CR, Dinsmore WW, Donnelly CM. Incidence of proximal renal tubular dysfunction in patients on tenofovir disoproxil fumarate. Int J STD AIDS. 2010; 21:150–151.
Article5. Ratcliffe L, Beadsworth MB, Pennell A, Phillips M, Vilar FJ. Managing hepatitis B/HIV coinfected: adding entecavir to truva-da (tenofovir disoproxil/emtricitabine) experienced patients. AIDS. 2011; 25:1051–1056.
Article6. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011; 57:773–780.
Article7. Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in ‘real-life'settings: from clinical trials to clinical practice. J Viral Hepat. 2012; 19:377–386.8. DeFronzo RA, Colvin OM, Braine H, Robertson GL, Davis PJ. Proceedings: Cyclophosphamide and the kidney. Cancer. 1974; 33:483–491.9. Philips FS, Sternberg SS, Cronin AP, Vidal PM. Cyclophosphamide and urinary bladder toxicity. Cancer Res. 1961; 21:1577–1589.10. Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA. Mitochondrial tubulopathy in tenofovir disoproxil fuma-rate-treated rats. J Acquir Immune Defic Syndr. 2009; 51:258–263.
Article11. Duarte-Rojo A, Heathcote EJ. Efficacy and safety of tenofovir disoproxil fumarate in patients with chronic hepatitis B. Therap Adv Gastroenterol. 2010; 3:107–119.12. Van Rompay KK, Brignolo LL, Meyer DJ, et al. Biological effects of short-term or prolonged administration of 9-[2-(phosphono-methoxy)propyl]adenine (tenofovir) to newborn and infant rhe-sus macaques. Antimicrob Agents Chemother. 2004; 48:1469–1487.
Article13. Wanner DP, Tyndall A, Walker UA. Tenofovir-induced osteomalacia. Clin Exp Rheumatol. 2009; 27:1001–1003.14. Perrot S, Aslangul E, Szwebel T, Caillat-Vigneron N, Le Jeunne C. Bone pain due to fractures revealing osteomalacia related to te-nofovir-induced proximal renal tubular dysfunction in a human immunodeficiency virus-infected patient. J Clin Rheumatol. 2009; 15:72–74.
Article15. Conti F, Vitale G, Cursaro C, Bernardi M, Andreone P. Tenofovir-induced Fanconi syndrome in a patient with chronic hepatitis B monoinfection. Ann Hepatol. 2016; 15:273–276.16. Hwang HS, Park CW, Song MJ. Tenofovir-associated Fanconi syndrome and nephrotic syndrome in a patient with chronic hepatitis B monoinfection. Hepatology. 2015; 62:1318–1320.
Article17. Magalhães-Costa P, Matos L, Barreiro P, Chagas C. Fanconi syndrome and chronic renal failure in a chronic hepatitis B monoinfected patient treated with tenofovir. Rev Esp Enferm Dig. 2015; 107:512–514.18. Viganò M, Brocchieri A, Spinetti A, et al. Tenofovir-induced Fanconi syndrome in chronic hepatitis B monoinfected patients that reverted after tenofovir withdrawal. J Clin Virol. 2014; 61:600–603.
Article19. Buti M, Tsai N, Petersen J, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015; 60:1457–1464.
Article20. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016; 63:261–283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fanconi Syndrome Associated with Long-term Adefovir and Subsequent Tenofovir Therapy for Chronic Hepatitis B Infection
- Bone Scintigraphy and Tenofovir-Induced Osteomalacia in Chronic Hepatitis B
- Fanconi's Syndrome Associated with Prolonged Adefovir Dipivoxil Therapy in a Hepatitis B Virus Patient
- Is tenofovir monotherapy a sufficient defense line against multi-drug resistant hepatitis B virus?
- A case of Fanconi syndrome