Immune Netw.
2017 Jun;17(3):133-143. 10.4110/in.2017.17.3.133.
The Role of Lipids in Development of Allergic Responses
- Affiliations
-
- 1Department of Cell Biology, Complutense University School of Medicine, Madrid 28040, Spain.
- 2Department of Microbiology and Immunology, Complutense University School of Medicine, Madrid 28040, Spain. emnaves@med.ucm.es
- KMID: 2383351
- DOI: http://doi.org/10.4110/in.2017.17.3.133
Abstract
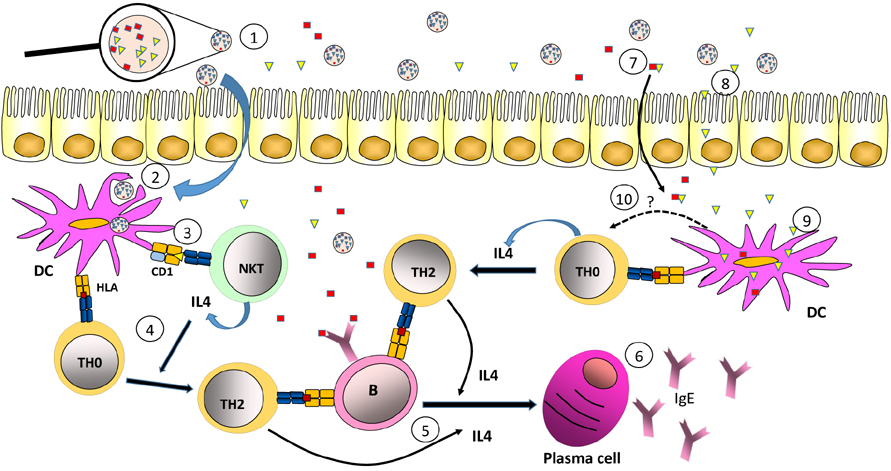
- Most allergic diseases are caused by activation of Th2 type immune responses resulting in the production of specific IgE against proteins found in normally harmless substances such as pollen, mites, epithelia or food. Allergenic substances are composed, in addition to proteins, of other compounds such as carbohydrates and lipids. Those lipids are able to promote the development of Th2-type responses associated with allergy. There are lipids found in pollen, milk or insect venom that are specifically recognized by CD1 restricted unconventional T lymphocytes, which can promote allergic reactions. Furthermore, a large number of allergens are proteins containing hydrophobic parts that specifically bind lipids that are capable to favor allergenic immune responses. Also, lipids associated to substances like pollen, dander, epithelia or the bacteria can act on cells of the innate system, including dendritic cells, which in turn lead to the differentiation of Th2-type clones. Finally, lipids may also influence the ability of allergens to be exposed to the immune system within the oral, respiratory or intestinal mucosa where allergic response occurs with great frequency.
Keyword
MeSH Terms
Figure
Reference
-
1. Dall'antonia F, Pavkov-Keller T, Zangger K, Keller W. Structure of allergens and structure based epitope predictions. Methods. 2014; 66:3–21.2. Matzinger P. The danger model: a renewed sense of self. Science. 2002; 296:301–305.
Article3. Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. 2014; 14:247–259.
Article4. Agea E, Russano A, Bistoni O, Mannucci R, Nicoletti I, Corazzi L, Postle AD, De LG, Porcelli SA, Spinozzi F. Human CD1-restricted T cell recognition of lipids from pollens. J Exp Med. 2005; 202:295–308.
Article5. Sasai T, Hirano Y, Maeda S, Matsunaga I, Otsuka A, Morita D, Nishida R, Nakayama H, Kuwahara Y, Sugita M, Mori N. Induction of allergic contact dermatitis by astigmatid mite-derived monoterpene, alpha-acaridial. Biochem Biophys Res Commun. 2008; 375:336–340.
Article6. Oteo M, Parra JF, Mirones I, Gimenez LI, Setien F, Martinez-Naves E. Single strand conformational polymorphism analysis of human CD1 genes in different ethnic groups. Tissue Antigens. 1999; 53:545–550.
Article7. Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol. 2015; 15:643–654.
Article8. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007; 25:297–336.
Article9. Birkholz AM, Kronenberg M. Antigen specificity of invariant natural killer T-cells. Biomed J. 2015; 38:470–483.
Article10. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997; 278:1626–1629.
Article11. Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005; 434:520–525.
Article12. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005; 434:525–529.
Article13. Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, Porcelli SA, Spath GF. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004; 200:895–904.
Article14. Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998; 279:1541–1544.
Article15. Zhou D, Mattner J, Cantu C III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004; 306:1786–1789.
Article16. Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000; 12:211–221.
Article17. Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003; 198:173–181.
Article18. Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011; 12:1202–1211.
Article19. Van Kaer L, Parekh VV, Wu L. The response of CD1d-restricted invariant NKT cells to microbial pathogens and their products. Front Immunol. 2015; 6:226.
Article20. Subleski JJ, Jiang Q, Weiss JM, Wiltrout RH. The split personality of NKT cells in malignancy, autoimmune and allergic disorders. Immunotherapy. 2011; 3:1167–1184.
Article21. Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006; 354:1117–1129.
Article22. Pham-Thi N, de BJ, Leite-de-Moraes MC. Invariant natural killer T cells in bronchial asthma. N Engl J Med. 2006; 354:2613–2616.
Article23. Reynolds C, Barkans J, Clark P, Kariyawasam H, Altmann D, Kay B, Boyton R. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J Allergy Clin Immunol. 2009; 124:860–862.
Article24. Koh YI, Shim JU. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol. 2010; 153:239–248.
Article25. Hamzaoui A, Cheik RS, Grairi H, Abid H, Ammar J, Chelbi H, Hamzaoui K. NKT cells in the induced sputum of severe asthmatics. Mediators Inflamm. 2006; 2006:71214.
Article26. Thomas SY, Lilly CM, Luster AD. Invariant natural killer T cells in bronchial asthma. N Engl J Med. 2006; 354:2613–2616.
Article27. Mutalithas K, Croudace J, Guillen C, Siddiqui S, Thickett D, Wardlaw A, Lammas D, Brightling C. Bronchoalveolar lavage invariant natural killer T cells are not increased in asthma. J Allergy Clin Immunol. 2007; 119:1274–1276.
Article28. Bratke K, Julius P, Virchow JC. Invariant natural killer T cells in obstructive pulmonary diseases. N Engl J Med. 2007; 357:194–195.
Article29. Scanlon ST, Thomas SY, Ferreira CM, Bai L, Krausz T, Savage PB, Bendelac A. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J Exp Med. 2011; 208:2113–2124.
Article30. Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003; 9:582–588.
Article31. Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006; 103:2782–2787.
Article32. Lisbonne M, Diem S, de Castro KA, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, Van EP, Dy M, Askenase P, Russo M, Vargaftig BB, Herbelin A, Leite-de-Moraes MC. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003; 171:1637–1641.
Article33. Nie H, Yang Q, Zhang G, Wang A, He Q, Liu M, Li P, Yang J, Huang Y, Ding X, Yu H, Hu S. Invariant NKT cells act as an adjuvant to enhance Th2 inflammatory response in an OVA-induced mouse model of asthma. PLoS One. 2015; 10:e0119901.
Article34. Abos-Gracia B, Lopez-Relano J, Revilla A, Castro L, Villalba M, Martin AB, Regueiro JR, Fernandez-Malave E, Martinez-Naves E, Gomez del. Human invariant natural killer T cells respond to antigen-presenting cells exposed to lipids from Olea europaea pollen. Int Arch Allergy Immunol. 2017; 173:12–22.
Article35. Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, DeKruyff RH, Savage PB, Umetsu DT. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013; 19:1297–1304.
Article36. Mirotti L, Florsheim E, Rundqvist L, Larsson G, Spinozzi F, Leite-de-Moraes M, Russo M, Alcocer M. Lipids are required for the development of Brazil nut allergy: the role of mouse and human iNKT cells. Allergy. 2013; 68:74–83.
Article37. Jyonouchi S, Abraham V, Orange JS, Spergel JM, Gober L, Dudek E, Saltzman R, Nichols KE, Cianferoni A. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J Allergy Clin Immunol. 2011; 128:102–109.
Article38. Jyonouchi S, Smith CL, Saretta F, Abraham V, Ruymann KR, Modayur-Chandramouleeswaran P, Wang ML, Spergel JM, Cianferoni A. Invariant natural killer T cells in children with eosinophilic esophagitis. Clin Exp Allergy. 2014; 44:58–68.
Article39. Bourgeois EA, Subramaniam S, Cheng TY, De JA, Layre E, Ly D, Salimi M, Legaspi A, Modlin RL, Salio M, Cerundolo V, Moody DB, Ogg G. Bee venom processes human skin lipids for presentation by CD1a. J Exp Med. 2015; 212:149–163.
Article40. Subramaniam S, Aslam A, Misbah SA, Salio M, Cerundolo V, Moody DB, Ogg G. Elevated and crossresponsive CD1a-reactive T cells in bee and wasp venom allergic individuals. Eur J Immunol. 2016; 46:242–252.
Article41. Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012; 129:1187–1197.
Article42. Behrendt H, Kasche A, Ebner von EC, Risse U, Huss-Marp J, Ring J. Secretion of proinflammatory eicosanoid-like substances precedes allergen release from pollen grains in the initiation of allergic sensitization. Int Arch Allergy Immunol. 2001; 124:121–125.
Article43. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharideenhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002; 196:1645–1651.
Article44. Plotz SG, Traidl-Hoffmann C, Feussner I, Kasche A, Feser A, Ring J, Jakob T, Behrendt H. Chemotaxis and activation of human peripheral blood eosinophils induced by pollen-associated lipid mediators. J Allergy Clin Immunol. 2004; 113:1152–1160.
Article45. Karg K, Dirsch VM, Vollmar AM, Cracowski JL, Laporte F, Mueller MJ. Biologically active oxidized lipids (phytoprostanes) in the plant diet and parenteral lipid nutrition. Free Radic Res. 2007; 41:25–37.
Article46. Traidl-Hoffmann C, Kasche A, Jakob T, Huger M, Plotz S, Feussner I, Ring J, Behrendt H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J Allergy Clin Immunol. 2002; 109:831–838.
Article47. Gutermuth J, Bewersdorff M, Traidl-Hoffmann C, Ring J, Mueller MJ, Behrendt H, Jakob T. Immunomodulatory effects of aqueous birch pollen extracts and phytoprostanes on primary immune responses in vivo. J Allergy Clin Immunol. 2007; 120:293–299.
Article48. Mariani V, Gilles S, Jakob T, Thiel M, Mueller MJ, Ring J, Behrendt H, Traidl-Hoffmann C. Immunomodulatory mediators from pollen enhance the migratory capacity of dendritic cells and license them for Th2 attraction. J Immunol. 2007; 178:7623–7631.
Article49. Kamijo S, Takai T, Kuhara T, Tokura T, Ushio H, Ota M, Harada N, Ogawa H, Okumura K. Cupressaceae pollen grains modulate dendritic cell response and exhibit IgEinducing adjuvant activity in vivo. J Immunol. 2009; 183:6087–6094.
Article50. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004; 5:987–995.
Article51. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.
Article52. Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007; 178:5375–5382.
Article53. Herre J, Gronlund H, Brooks H, Hopkins L, Waggoner L, Murton B, Gangloff M, Opaleye O, Chilvers ER, Fitzgerald K, Gay N, Monie T, Bryant C. Allergens as immunomodulatory proteins: the cat dander protein Fel d 1 enhances TLR activation by lipid ligands. J Immunol. 2013; 191:1529–1535.
Article54. Mueller GA, Pedersen LC, Lih FB, Glesner J, Moon AF, Chapman MD, Tomer KB, London RE, Pomes A. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol. 2013; 132:1420–1426.
Article55. Mittag D, Varese N, Scholzen A, Mansell A, Barker G, Rice G, Rolland JM, O'Hehir RE. TLR ligands of ryegrass pollen microbial contaminants enhance Th1 and Th2 responses and decrease induction of Foxp3(hi) regulatory T cells. Eur J Immunol. 2013; 43:723–733.
Article56. Heydenreich B, Bellinghausen I, Konig B, Becker WM, Grabbe S, Petersen A, Saloga J. Gram-positive bacteria on grass pollen exhibit adjuvant activity inducing inflammatory T cell responses. Clin Exp Allergy. 2012; 42:76–84.
Article57. Abos-Gracia B, del Moral MG, Lopez-Relano J, Viana-Huete V, Castro L, Villalba M, Martinez-Naves E. Olea europaea pollen lipids activate invariant natural killer T cells by upregulating CD1d expression on dendritic cells. J Allergy Clin Immunol. 2013; 131:1393–1399.58. Brewer JM, Pollock KG, Tetley L, Russell DG. Vesicle size influences the trafficking, processing, and presentation of antigens in lipid vesicles. J Immunol. 2004; 173:6143–6150.
Article59. Fernandes H, Michalska K, Sikorski M, Jaskolski M. Structural and functional aspects of PR-10 proteins. FEBS J. 2013; 280:1169–1199.
Article60. Bashir ME, Lui JH, Palnivelu R, Naclerio RM, Preuss D. Pollen lipidomics: lipid profiling exposes a notable diversity in 22 allergenic pollen and potential biomarkers of the allergic immune response. PLoS One. 2013; 8:e57566.
Article61. Martin-Pedraza L, Gonzalez M, Gomez F, Blanca-Lopez N, Garrido-Arandia M, Rodriguez R, Torres MJ, Blanca M, Villalba M, Mayorga C. Two nonspecific lipid transfer proteins (nsLTPs) from tomato seeds are associated to severe symptoms of tomato-allergic patients. Mol Nutr Food Res. 2016; 60:1172–1182.
Article62. Kleine-Tebbe J, Hamilton RG. Cashew allergy, 2S albumins, and risk predictions based on IgE antibody levels. Allergy. 2017; 72:515–518.
Article63. Seutter von Loetzen C, Hoffmann T, Hartl MJ, Schweimer K, Schwab W, Rosch P, Hartl-Spiegelhauer O. Secret of the major birch pollen allergen Bet v 1: identification of the physiological ligand. Biochem J. 2014; 457:379–390.
Article64. Hilger C, Kuehn A, Hentges F. Animal lipocalin allergens. Curr Allergy Asthma Rep. 2012; 12:438–447.
Article65. Thomas WR, Hales BJ, Smith WA. Genetically engineered vaccines. Curr Allergy Asthma Rep. 2005; 5:197–203.
Article66. Roth-Walter F, Pacios LF, Gomez-Casado C, Hofstetter G, Roth GA, Singer J, az-Perales A, Jensen-Jarolim E. The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron. PLoS One. 2014; 9:e104803.
Article67. Roth-Walter F, Gomez-Casado C, Pacios LF, Mothes-Luksch N, Roth GA, Singer J, az-Perales A, Jensen-Jarolim E. Bet v 1 from birch pollen is a lipocalin-like protein acting as allergen only when devoid of iron by promoting Th2 lymphocytes. J Biol Chem. 2014; 289:17416–17421.
Article68. Egger M, Hauser M, Mari A, Ferreira F, Gadermaier G. The role of lipid transfer proteins in allergic diseases. Curr Allergy Asthma Rep. 2010; 10:326–335.
Article69. Yeats TH, Rose JK. The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs). Protein Sci. 2008; 17:191–198.
Article70. Agati G, Brunetti C, Di FM, Ferrini F, Pollastri S, Tattini M. Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol Biochem. 2013; 72:35–45.
Article71. Akkerdaas JH, Schocker F, Vieths S, Versteeg S, Zuidmeer L, Hefle SL, Aalberse RC, Richter K, Ferreira F, van RR. Cloning of oleosin, a putative new hazelnut allergen, using a hazelnut cDNA library. Mol Nutr Food Res. 2006; 50:18–23.
Article72. Leduc V, Moneret-Vautrin DA, Tzen JT, Morisset M, Guerin L, Kanny G. Identification of oleosins as major allergens in sesame seed allergic patients. Allergy. 2006; 61:349–356.
Article73. Angelina A, Sirvent S, Palladino C, Vereda A, Cuesta-Herranz J, Eiwegger T, Rodriguez R, Breiteneder H, Villalba M, Palomares O. The lipid interaction capacity of Sin a 2 and Ara h 1, major mustard and peanut allergens of the cupin superfamily, endorses allergenicity. Allergy. 2016; 71:1284–1294.
Article74. Petersen A, Rennert S, Kull S, Becker WM, Notbohm H, Goldmann T, Jappe U. Roasting and lipid binding provide allergenic and proteolytic stability to the peanut allergen Ara h 8. Biol Chem. 2014; 395:239–250.
Article75. Salcedo G, Sanchez-Monge R, Barber D, Diaz-Perales A. Plant non-specific lipid transfer proteins: an interface between plant defence and human allergy. Biochim Biophys Acta. 2007; 1771:781–791.
Article76. Pacios LF, Gomez-Casado C, Tordesillas L, Palacin A, Sanchez-Monge R, Diaz-Perales A. Computational study of ligand binding in lipid transfer proteins: Structures, interfaces, and free energies of protein-lipid complexes. J Comput Chem. 2012; 33:1831–1844.
Article77. Tordesillas L, Gomez-Casado C, Garrido-Arandia M, Murua-Garcia A, Palacin A, Varela J, Konieczna P, Cuesta-Herranz J, Akdis CA, O'Mahony L, Diaz-Perales A. Transport of Pru p 3 across gastrointestinal epithelium - an essential step towards the induction of food allergy. Clin Exp Allergy. 2013; 43:1374–1383.
Article78. Yin SC, Liao EC, Chiu CL, Chang CY, Tsai JJ. Der p2 Internalization by epithelium synergistically augments toll-like receptor-mediated proinflammatory signaling. Allergy Asthma Immunol Res. 2015; 7:393–403.
Article79. Mattila K, Renkonen R. Modelling of Bet v 1 binding to lipids. Scand J Immunol. 2009; 70:116–124.
Article80. Li J, Wang Y, Tang L, de Villiers WJ, Cohen D, Woodward J, Finkelman FD, Eckhardt ER. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J Allergy Clin Immunol. 2013; 131:442–450.
Article81. Wang TY, Liu M, Portincasa P, Wang DQ. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest. 2013; 43:1203–1223.
Article82. Sancho AI, Wangorsch A, Jensen BM, Watson A, Alexeev Y, Johnson PE, Mackie AR, Neubauer A, Reese G, Ballmer-Weber B, Hoffmann-Sommergruber K, Skov PS, Vieths S, Mills EN. Responsiveness of the major birch allergen Bet v 1 scaffold to the gastric environment: impact on structure and allergenic activity. Mol Nutr Food Res. 2011; 55:1690–1699.
Article83. Vassilopoulou E, Rigby N, Moreno FJ, Zuidmeer L, Akkerdaas J, Tassios I, Papadopoulos NG, Saxoni-Papageorgiou P, van Ree R, Mills C. Effect of in vitro gastric and duodenal digestion on the allergenicity of grape lipid transfer protein. J Allergy Clin Immunol. 2006; 118:473–480.
Article84. Bossios A, Theodoropoulou M, Mondoulet L, Rigby NM, Papadopoulos NG, Bernard H, del-Patient K, Wal JM, Mills CE, Papageorgiou P. Effect of simulated gastro-duodenal digestion on the allergenic reactivity of betalactoglobulin. Clin Transl Allergy. 2011; 1:6–11.
Article85. Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, von Mutius E, Chanez P, Lambrecht BN, Hammad H. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015; 349:1106–1110.
Article