Diabetes Metab J.
2016 Feb;40(1):46-53. 10.4093/dmj.2016.40.1.46.
Protective Effects of Ginger (Zingiber officinale) Extract against Diabetes-Induced Heart Abnormality in Rats
- Affiliations
-
- 1Department of Pathology, Urmia University of Medical Sciences Faculty of Medicine, Urmia, Iran.
- 2Department of Physiology, Urmia University of Medical Sciences Faculty of Medicine, Urmia, Iran. ashirpoor@yahoo.com
- 3Department of Biochemistry, Urmia University of Medical Sciences Faculty of Medicine, Urmia, Iran.
- KMID: 2383314
- DOI: http://doi.org/10.4093/dmj.2016.40.1.46
Abstract
- BACKGROUND
Diabetic cardiomyopathy is an important causal factor in morbidity and mortality among diabetic patients, and currently, no effective means are available to reverse its pathological progress. The purpose of the present study was to investigate the effect of ginger extract on apolipoproteins (apo) A and B, hyperhomocysteinemia, cathepsin G and leptin changes, as well as cardiac fibrosis and heart muscle cell proliferation under hyperglycemic conditions in vivo.
METHODS
Twenty-four male Wistar rats were divided into three groups, namely: control, non-treated diabetic, and ginger extract-treated diabetic groups. The ginger extract-treated diabetic group received a 50 mg daily dose of ginger extract intragastrically for 6 weeks.
RESULTS
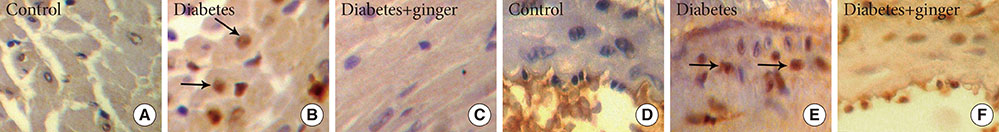
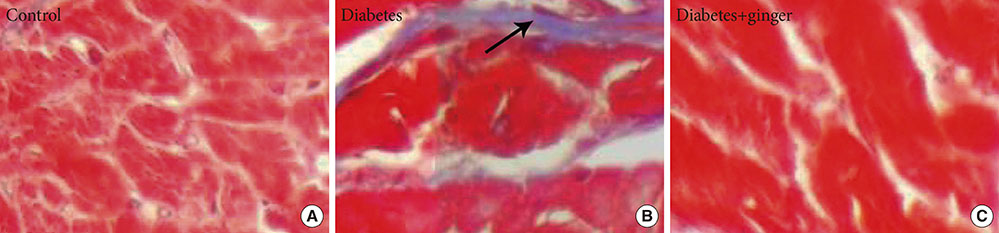
The results revealed concurrent significant increases in plasma C-reactive protein (CRP), homocysteine (Hcy), cathepsin G and apoB levels and decreases in apoA and leptin levels in the non-treated diabetic group compared to the control group. Moreover, heart structural changes, including fibrosis and heart muscle cell proliferation, were observed in non-treated diabetic rats compared to the control rats. Significant amelioration of changes in the heart structure together with restoration of the elevated levels of Hcy and CRP, leptin, cathepsin G, and apoA and B were found in the ginger extract-treated diabetic group compared to the non-treated diabetic group.
CONCLUSION
The findings indicated that ginger extract significantly reduces heart structural abnormalities in diabetic rats and that these effects might be associated with improvements in serum apo, leptin, cathepsin G, and Hcy levels and with the antioxidant properties of ginger extract.
MeSH Terms
-
Animals
Apolipoproteins A
Apolipoproteins B
C-Reactive Protein
Cathepsin G
Diabetic Cardiomyopathies
Fibrosis
Ginger*
Heart Defects, Congenital*
Heart*
Homocysteine
Humans
Hyperhomocysteinemia
Leptin
Male
Mortality
Myocytes, Cardiac
Plasma
Rats*
Rats, Wistar
Apolipoproteins A
Apolipoproteins B
C-Reactive Protein
Cathepsin G
Homocysteine
Leptin
Figure
Reference
-
1. Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006; 98:596–605.2. Shirpoor A, Salami S, Khadem-Ansari MH, Ilkhanizadeh B, Pakdel FG, Khademvatani K. Cardioprotective effect of vitamin E: rescues of diabetes-induced cardiac malfunction, oxidative stress, and apoptosis in rat. J Diabetes Complications. 2009; 23:310–316.3. Zhao J, Randive R, Stewart JA. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diabetes. 2014; 5:860–867.4. Okatan EN, Tuncay E, Turan B. Cardioprotective effect of selenium via modulation of cardiac ryanodine receptor calcium release channels in diabetic rat cardiomyocytes through thioredoxin system. J Nutr Biochem. 2013; 24:2110–2118.5. Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, Unger T, Schultheiss HP, Pauschinger M, Tschope C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007; 56:641–646.6. Jahanyar J, Youker KA, Loebe M, Assad-Kottner C, Koerner MM, Torre-Amione G, Noon GP. Mast cell-derived cathepsin g: a possible role in the adverse remodeling of the failing human heart. J Surg Res. 2007; 140:199–203.7. Reilly CF, Tewksbury DA, Schechter NM, Travis J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem. 1982; 257:8619–8622.8. Soderberg S, Stegmayr B, Ahlbeck-Glader C, Slunga-Birgander L, Ahren B, Olsson T. High leptin levels are associated with stroke. Cerebrovasc Dis. 2003; 15:63–69.9. Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: does leptin have a role? J Am Coll Cardiol. 2011; 58:1870–1877.10. Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: the Heart and Soul Study. Atherosclerosis. 2011; 217:503–508.11. Hou N, Luo JD. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011; 38:905–913.12. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010; 464:1293–1300.13. Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J Biol Chem. 2009; 284:27090–27100.14. Utriainen T, Malmstrom R, Makimattila S, Yki-Jarvinen H. Supraphysiological hyperinsulinemia increases plasma leptin concentrations after 4 h in normal subjects. Diabetes. 1996; 45:1364–1366.15. Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF. Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro. Diabetes. 1996; 45:699–701.16. Boden G, Chen X, Kolaczynski JW, Polansky M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest. 1997; 100:1107–1113.17. Nile SH, Park SW. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind Crops Prod. 2015; 70:238–244.18. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988; 41:467–470.19. Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006; 259:437–446.20. Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy: a review of the evidence. J Intern Med. 2006; 259:493–519.21. Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004; 81:223–241.22. Kalra SP. Central leptin insufficiency syndrome: an interactive etiology for obesity, metabolic and neural diseases and for designing new therapeutic interventions. Peptides. 2008; 29:127–138.23. Gutierrez-Juarez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004; 279:49704–49715.24. Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003; 93:277–279.25. Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000; 49:293–297.26. Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002; 51:168–173.27. Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010; 7:22–29.28. Guzman-Ruiz R, Somoza B, Gil-Ortega M, Merino B, Cano V, Attane C, Castan-Laurell I, Valet P, Fernandez-Alfonso MS, Ruiz-Gayo M. Sensitivity of cardiac carnitine palmitoyltransferase to malonyl-CoA is regulated by leptin: similarities with a model of endogenous hyperleptinemia. Endocrinology. 2010; 151:1010–1018.29. Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008; 88:389–419.30. McGaffin KR, Witham WG, Yester KA, Romano LC, O'Doherty RM, McTiernan CF, O'Donnell CP. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res. 2011; 89:60–71.31. Schram K, Sweeney G. Implications of myocardial matrix remodeling by adipokines in obesity-related heart failure. Trends Cardiovasc Med. 2008; 18:199–205.32. Zibadi S, Cordova F, Slack EH, Watson RR, Larson DF. Leptin's regulation of obesity-induced cardiac extracellular matrix remodeling. Cardiovasc Toxicol. 2011; 11:325–333.33. Shirpoor A, Norouzi L, Khadem Ansari MH, Ilkhanizadeh B, Gharaaghaji R. Vasoprotective effect of vitamin E: rescue of ethanol-induced atherosclerosis and inflammatory stress in rat vascular wall. Int Immunopharmacol. 2013; 16:498–504.34. Passino C, Barison A, Vergaro G, Gabutti A, Borrelli C, Emdin M, Clerico A. Markers of fibrosis, inflammation, and remodeling pathways in heart failure. Clin Chim Acta. 2015; 443:29–38.35. Shirpoor A, Nemati S, Ansari MH, Ilkhanizadeh B. The protective effect of vitamin E against prenatal and early postnatal ethanol treatment-induced heart abnormality in rats: a 3-month follow-up study. Int Immunopharmacol. 2015; 26:72–79.36. Shirpoor A, Khadem Ansari MH, Heshmatian B, Ilkhanizadeh B, Noruzi L, Abdollahzadeh N, Saboory E. Decreased blood pressure with a corresponding decrease in adhesive molecules in diabetic rats caused by vitamin E administration. J Diabetes. 2012; 4:362–368.37. Alireza S, Leila N, Siamak S, Mohammad-Hasan KA, Behrouz I. Effects of vitamin E on pathological changes induced by diabetes in rat lungs. Respir Physiol Neurobiol. 2013; 185:593–599.38. Taghizadeh Afshari A, Shirpoor A, Farshid A, Saadatian R, Rasmi Y, Saboory E, Ilkhanizadeh B, Allameh A. The effect of ginger on diabetic nephropathy, plasma antioxidant capacity and lipid peroxidation in rats. Food Chem. 2007; 101:148–153.39. Shanmugam KR, Mallikarjuna K, Nishanth K, Kuo CH, Sathyavelu Reddy K. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011; 124:1436–1442.40. Ramudu SK, Korivi M, Kesireddy N, Chen CY, Kuo CH, Kesireddy SR. Ginger feeding protects against renal oxidative damage caused by alcohol consumption in rats. J Ren Nutr. 2011; 21:263–270.41. Isa Y, Miyakawa Y, Yanagisawa M, Goto T, Kang MS, Kawada T, Morimitsu Y, Kubota K, Tsuda T. 6-Shogaol and 6-gingerol, the pungent of ginger, inhibit TNF-alpha mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2008; 373:429–434.42. Srivastava KC, Mustafa T. Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders. Med Hypotheses. 1992; 39:342–348.43. German JP, Wisse BE, Thaler JP, Oh IS, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky GJ Jr, Schwartz MW, Morton GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010; 59:1626–1634.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Zingiber Officinale Roscoe Extracts on Mice Immune Cell Activation
- Steamed Ginger Extract Exerts Anti-inflammatory Effects in Helicobacter pylori-infected Gastric Epithelial Cells through Inhibition of NF-κB
- Enhancing Effect of Zingiber Officinale Roscoe Extracts on Mouse Spleen and Macrophage Cells Activation
- Systematic review of the effect of dried ginger powder on improvement of nausea and vomiting associated with early pregnancy or motion sickness
- Neuroprotective effect of fermented ginger extracts by Bacillus subtilis in SH-SY5Y cells